Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide) †
Abstract
:1. Introduction
2. Results and Discussion
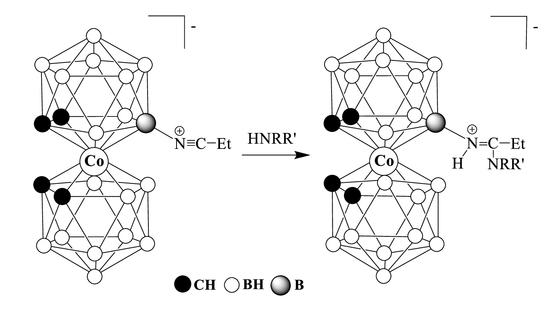
2.1. Nucleophilic Addition of Primary Amines
2.2. Nucleophilic Addition of Secondary Amines
2.3. X-ray Diffraction Study
3. Conclusions
4. Experimental
4.1. Materials and Methods
4.2. General Procedure for Synthesis of Compounds 1–10
4.3. Single Crystal X-ray Diffraction Study
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawthorne, M.F.; Andrews, T.D. Carborane analogues of cobalticinium ion. J. Chem. Soc. Chem. Commun. 1965, 19, 443–444. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Young, D.; Andrews, T.D.; Hove, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjes, M.; Warren, L.F.; Wegner, P.A. π-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czechoslov. Chem. Commun. 2002, 64, 783–805. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849–850, 170–194. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Clingerman, D.J.; Morris, W.; Wilmer, C.E.; Sarjeant, A.A.; Stern, C.L.; O’Keeffe, M.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K.; et al. Metallacarborane-based metal-organic framework with a complex topology. Cryst. Growth Des. 2014, 14, 1324–1330. [Google Scholar] [CrossRef]
- Tarres, M.; Arderiu, V.S.; Zaulet, A.; Viñas, C.; Fabrizi de Biani, F.; Teixidor, F. How to get the desired reduction voltage in a single framework! Metallacarborane as an optimal probe for sequential voltage tuning. Dalton Trans. 2015, 44, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Buades, A.B.; Arderiu, V.S.; Olid-Britos, D.; Viñas, C.; Sillanpää, R.; Haukka, M.; Fontrodona, X.; Paradinas, M.; Ocal, C.; Teixidor, F. Electron accumulative molecules. J. Am. Chem. Soc. 2018, 140, 2957–2970. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Tetrathiafulvalene-based radical cation salts with transition metal bis(dicarbollide) anions. CrystEngComm 2015, 17, 4754–4767. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kosenko, I.D.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Electroconducting radical-cation salts based on tetrathiafulvalene derivatives and transition metal bis(dicarbollides). Russ. J. Gen. Chem. 2019, 89, 971–987. [Google Scholar] [CrossRef]
- Pichaandi, K.R.; Nilakantan, L.; Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Electronic interactions between ferrocenyl units facilitated by the cobalt bis(dicarbollide) anion linker: An experimental and DFT study. Eur. J. Inorg. Chem. 2017, 666–670. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 4444–4451. [Google Scholar] [CrossRef] [Green Version]
- Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. Bis(dicarbollide) complexes of transition metals as a platform for molecular switches. Study of complexation of 8,8′-bis(methylsulfanyl) derivatives of cobalt and iron bis(dicarbollides). Molecules 2020, 25, 5745. [Google Scholar] [CrossRef]
- Fuentes, I.; Garcıa-Mendiola, T.; Sato, S.; Pita, M.; Nakamura, H.; Lorenzo, E.; Teixidor, F.; Marques, F.; Viñas, C. Metallacarboranes on the road to anticancer therapies: Cellular uptake, DNA interaction, and biological evaluation of cobaltabisdicarbollide [COSAN]−. Chem. Eur. J. 2018, 24, 17239–17254. [Google Scholar] [CrossRef]
- Řezačova, P.; Cigler, P.; Matejiček, P.; Lepšik, M.; Pokorna, J.; Grüner, B.; Konvalinka, J. Medicinal application of carboranes: Inhibition of HIV protease. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 41–70. [Google Scholar] [CrossRef]
- Grüner, B.; Brynda, J.; Das, V.; Šicha, V.; Stepankova, J.; Nekvinda, J.; Holub, J.; Pospisilova, K.; Fabry, M.; Pachtl, P.; et al. Metallacarborane sulfamides: Unconventional, specific, and highly selective inhibitors of carbonic anhydrase IX. J. Med. Chem. 2019, 62, 9560–9575. [Google Scholar] [CrossRef] [PubMed]
- Grüner, B.; Kugler, M.; El Anwar, S.; Holub, J.; Nekvinda, J.; Bavol, D.; Růžičková, Z.; Pospíšilová, K.; Fábry, M.; Král, V.; et al. Cobalt bis(dicarbollide) alkylsulfonamides: Potent and highly selective inhibitors of tumor specific carbonic anhydrase IX. ChemPlusChem 2021, 86, 352–363. [Google Scholar] [CrossRef]
- Kugler, M.; Nekvinda, J.; Holub, J.; El Anwar, S.; Das, V.; Šícha, V.; Pospíšilová, K.; Fábry, M.; Král, V.; Brynda, J.; et al. Inhibitors of CA IX enzyme based on polyhedral boron compounds. ChemBioChem 2021, 22, 2741–2761. [Google Scholar] [CrossRef] [PubMed]
- Nekvinda, J.; Rozycka, D.; Rykowski, S.; Wyszko, E.; Fedoruk-Wyszomirska, A.; Gurda, D.; Orlicka-Płocka, M.; Giel-Pietraszuk, M.; Kiliszek, A.; Rypniewski, W.; et al. Synthesis of naphthalimide-carborane and metallacarborane conjugates: Anticancer activity, DNA binding ability. Bioorg. Chem. 2020, 94, 103432. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, A.B.; Nawrot, B.; Lesnikowski, Z.J. DNA modified with boron-metal cluster complexes [M(C2B9H11)2]-: Synthesis, properties, and applications. Int. J. Mol. Sci. 2018, 19, 3501. [Google Scholar] [CrossRef] [Green Version]
- Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Ulybina, O.V.; Tsiprovskiy, A.G.; Berzina, M.Y.; Lobanova, I.A.; Sivaev, I.B.; Bregadze, V.I.; Mironov, A.F. New conjugates of cobalt bis(dicarbollide) with chlorophyll a derivatives. Mendeleev Commun. 2011, 21, 84–86. [Google Scholar] [CrossRef]
- Efremenko, A.V.; Ignatova, A.A.; Grin, M.A.; Sivaev, I.B.; Mironov, A.F.; Bregadze, V.I.; Feofanov, A.V. Chlorin e6 fused with a cobalt-bis(dicarbollide) nanoparticle provides efficient boron delivery and photoinduced cytotoxicity in cancer cells. Photochem. Photobiol. Sci. 2014, 13, 92–102. [Google Scholar] [CrossRef]
- Volovetsky, A.B.; Sukhov, V.S.; Balalaeva, I.V.; Dudenkova, V.V.; Shilyagina, N.Y.; Feofanov, A.V.; Efremenko, A.V.; Grin, M.A.; Mironov, A.F.; Sivaev, I.B.; et al. Pharmacokinetics of chlorin e6-cobalt bis(dicarbollide) conjugate in balb/c mice with engrafted carcinoma. Int. J. Mol. Sci. 2017, 18, 2556. [Google Scholar] [CrossRef] [Green Version]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef] [PubMed]
- Druzina, A.A.; Shmalko, A.V.; Andreichuk, E.P.; Zhidkova, O.B.; Kosenko, I.D.; Semioshkin, A.; Sivaev, I.B.; Mandal, S.; Shen, Z.; Bregadze, V.I. ‘Click’ synthesis of cobalt bis(dicarbollide)–cholesterol conjugates. Mendeleev. Commun. 2019, 29, 628–630. [Google Scholar] [CrossRef]
- Dubey, R.D.; Sarkar, A.; Shen, Z.; Bregadze, V.I.; Sivaev, I.B.; Druzina, A.A.; Zhidkova, O.B.; Shmal’ko, A.V.; Kosenko, I.D.; Sreejyothi, P.; et al. Effects of linkers on the development of liposomal formulation of cholesterol conjugated cobalt bis(dicarbollides). J. Pharm. Sci. 2021, 110, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X. A highly potent antibacterial agent targeting methicillin-resistant Staphylococcus aureus based on cobalt bis(1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Swietnicki, W.; Goldeman, W.; Psurski, M.; Nasulewicz-Goldeman, A.; Boguszewska-Czubara, A.; Drab, M.; Sycz, J.; Goszczyński, T.M. Metallacarborane derivatives effective against Pseudomonas aeruginosa and Yersinia enterocolitica. Int. J. Mol. Sci. 2021, 22, 6762. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of cobalt and iron bis(dicarbollides) and their use in organic synthesis. Russ. Chem. Rev. 2021, 90, 785–830. [Google Scholar] [CrossRef]
- Van Dijk, T.; Slootweg, J.C.; Lammertsma, K. Nitrilium ions—Synthesis and applications. Org. Biomol. Chem. 2017, 15, 10134–10144. [Google Scholar] [CrossRef]
- Michelin, R.A.; Mozzon, M.; Bertani, R. Reactions of transition metal-coordinated nitriles. Coord. Chem. Rev. 1996, 147, 299–338. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Additions to metal-activated organonitriles. Chem. Rev. 2002, 102, 1771–1802. [Google Scholar] [CrossRef] [PubMed]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Metal-mediated and metal-catalyzed hydrolysis of nitriles. Inorg. Chim. Acta 2005, 358, 1–21. [Google Scholar] [CrossRef]
- Bokach, N.A.; Kukushkin, V.Y. Addition of HO-nucleophiles to free and coordinated nitriles. Russ. Chem. Rev. 2005, 74, 153–170. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-involving synthesis and reactions of oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium derivatives of polyhedral boron compounds (boranes, carboranes, metallocarboranes): Synthesis and reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Bykov, A.Y.; Kubasov, A.S.; Polyakova, I.N.; Razgonyaeva, G.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Hydrolysis of nitrilium derivatives of the closo-decaborate anion [2-B10H9(N≡CR)]- (R = CH3, C2H5, C(CH3)3, or C6H5). Russ. J. Inorg. Chem. 2017, 62, 468–475. [Google Scholar] [CrossRef]
- Voinova, V.V.; Selivanov, N.A.; Plyushchenko, I.V.; Vokuev, M.F.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Rodin, I.A.; et al. Fused 1,2-diboraoxazoles based on closo-decaborate anion—Novel members of diboroheterocycle class. Molecules 2021, 26, 248. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Klyukin, I.N.; Bykov, A.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition of alcohols to anionic [2-B10H9NCR]− (R=Et, t-Bu): An approach to producing new borylated imidates. Polyhedron 2017, 123, 176–183. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Polyakova, I.N.; Razgonyaeva, G.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Reactions of nucleophilic addition of primary amines to the nitrilium derivative of the closo-decaborate anion [2-B10H9(N≡CCH3)]−. Russ. J. Inorg. Chem. 2011, 56, 847–855. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Nelyubin, A.V.; Klyukin, I.N.; Selivanov, N.A.; Bortnikov, E.O.; Grigoriev, M.S.; Zhizhin, K.Yu.; Kuznetsov, N.T. Nucleophilic addition reaction of secondary amines to acetonitrilium closo-decaborate [2-B10H9NCCH3]−. Russ. J. Inorg. Chem. 2019, 64, 841–846. [Google Scholar] [CrossRef]
- Burianova, V.K.; Bolotin, D.S.; Mikhredov, A.S.; Novikov, A.S.; Mokolokolo, P.P.; Roodt, A.; Boyarskiy, V.P.; Dar’in, D.; Krasavin, M.; Suslonov, V.V.; et al. Mechanism of generation of closo-decaborato amidrazones. Intramolecular non-covalent B–H⋯π(Ph) interaction determines stabilization of the configuration around the amidrazone C=N bond. New J. Chem. 2018, 42, 8693–8703. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Burianova, V.K.; Novikov, A.S.; Demakova, M.Y.; Pretorius, C.; Mokolokolo, P.P.; Roodt, A.; Bokach, N.A.; Suslonov, V.V.; Zhdanov, A.P.; et al. Nucleophilicity of oximes based upon addition to a nitrilium closo-decaborate cluster. Organometallics 2016, 35, 3612–3623. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Demakova, M.Y.; Daines, E.A.; Avdontseva, M.S.; Zhdanov, A.P.; Zhizhin, K.Yu.; Kuznetsov, N.T. Nucleophilic addition of aromatic amide oximes to [2-B10H9NCC2H5]- anion. Russ. J. Gen. Chem. 2017, 87, 37–43. [Google Scholar] [CrossRef]
- Mindich, A.L.; Bokach, N.A.; Kuznetsov, M.L.; Haukka, M.; Zhdanov, A.P.; Zhizhin, K.Y.; Miltsov, S.A.; Kuznetsov, N.T.; Kukushkin, V.Y. Coupling of azomethine ylides with nitrilium derivatives of closo-decaborate clusters: A synthetic and theoretical study. ChemPlusChem 2012, 77, 1075–1086. [Google Scholar] [CrossRef]
- Daines, E.A.; Bolotin, D.S.; Bokach, N.A.; Gurzhiy, V.V.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Push-pull alkenes bearing closo-decaborate cluster generated via nucleophilic addition of carbanions to borylated nitrilium salts. Inorg. Chim. Acta 2018, 471, 372–376. [Google Scholar] [CrossRef]
- Mindich, A.L.; Bokach, N.A.; Kuznetsov, M.L.; Starova, G.L.; Zhdanov, A.P.; Zhizhin, K.Y.; Miltsov, S.A.; Kuznetsov, N.T.; Kukushkin, V.Y. Borylated tetrazoles from cycloaddition of azide anions to nitrilium derivatives of closo-decaborate clusters. Organometallics 2013, 32, 6576–6586. [Google Scholar] [CrossRef]
- Mindich, A.L.; Bokach, N.A.; Dolgushin, F.M.; Haukka, M.; Lisitsyn, L.A.; Zhdanov, A.P.; Zhizhin, K.Y.; Miltsov, S.A.; Kuznetsov, N.T.; Kukushkin, V.Y. 1,3-Dipolar cycloaddition of nitrones to a nitrile functionality in closo-decaborate clusters: A novel reactivity mode for the borylated C≡N group. Organometallics 2012, 31, 1716–1724. [Google Scholar] [CrossRef]
- Šícha, V.; Plešek, J.; Kvíčalová, M.; Císařová, I.; Grüner, B. Boron(8) substituted nitrilium and ammonium derivatives, versatile cobalt bis(1,2-dicarbollide) building blocks for synthetic purposes. Dalton Trans. 2009, 851–860. [Google Scholar] [CrossRef]
- El Anwar, S.; Růžičková, Z.; Bavol, D.; Fojt, L.; Grüner, B. Tetrazole Ring substitution at carbon and boron sites of the cobalt bis(dicarbollide) ion available via dipolar cycloadditions. Inorg. Chem. 2020, 59, 17430–17442. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.V.; Stogniy, M.Y.; Chekulaeva, L.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Grin, M.A.; Mironov, A.F.; Bregadze, V.I. Synthesis and reactivity of propionitrilium derivatives of cobalt and iron bis(dicarbollides). New J. Chem. 2020, 44, 15836–15848. [Google Scholar] [CrossRef]
- Shriner, R.; Neumann, F. The chemistry of the amidines. Chem. Rev. 1944, 35, 351–425. [Google Scholar] [CrossRef]
- Patai, S. (Ed.) The Chemistry of Amidines and Imidates, Vol. 1 (PATAI’S Chemistry of Functional Groups); John Wiley & Sons Ltd.: London, UK, 1975; 677p. [Google Scholar] [CrossRef]
- Patai, S.; Rappoport, Z. (Eds.) The Chemistry of Amidines and Imidates, Vol. 2 (PATAI’S Chemistry of Functional Groups); John Wiley & Sons Ltd.: Chichester, UK, 1991; 924p. [Google Scholar] [CrossRef]
- Granik, V.G. Advances in the chemistry of amidines. Russ. Chem. Rev. 1983, 52, 669–703. [Google Scholar] [CrossRef]
- Aly, A.A.; Bräse, S.; Gomaa, M.A.-M. Amidines: Their synthesis, reactivity, and applications in heterocycle synthesis. ARKIVOC 2018, 85–138. [Google Scholar] [CrossRef]
- Chen, X.M.; Orser, B.A.; MacDonald, J.F. Design and screening of ASIC inhibitors based on aromatic diamidines for combating neurological disorders. Eur. J. Pharm. 2010, 648, 15–23. [Google Scholar] [CrossRef]
- Kotthaus, J.; Steinmetzer, T.; van de Locht, A.; Clement, B. Analysis of highly potent amidine containing inhibitors of serine proteases and their N-hydroxylated prodrugs (amidoximes). J. Enzyme Inhibition Med. Chem. 2011, 26, 115–122. [Google Scholar] [CrossRef]
- Huang, T.L.; Mayence, A.; Vanden Eynde, J.J. Some non-conventional biomolecular targets for diamidines. A short survey. Bioorg. Med. Chem. 2014, 22, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Krstulović, L.; Stolić, I.; Jukić, M.; Opačak-Bernardi, T.; Starčević, K.; Bajić, M.; Glavaš-Obrovac, L. New quinoline-arylamidine hybrids: Synthesis, DNA/RNA binding and antitumor activity. Eur. J. Med. Chem. 2017, 137, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Rastija, V.; Jukić, M.; Opačak-Bernardi, T.; Krstulović, L.; Stolić, I.; Glavaš-Obrovac, L.; Bajić, M. Investigation of the structural and physicochemical requirements of quinoline-arylamidine hybrids for the growth inhibition of K562 and Raji leukemia cells. Turk. J. Chem. 2019, 43, 251–265. [Google Scholar] [CrossRef]
- Yadava, U.; Yadav, S.K.; Yadav, R.K. Investigations on bisamidine derivatives as novel minor groove binders with the dodecamer 5′(CGCGAATTCGCG)3′. J. Mol. Liq. 2019, 280, 135–152. [Google Scholar] [CrossRef]
- Behrouz, S.; Kühl, N.; Klein, C.D. A facile approach towards amidinophenylalanine derivatives as building blocks for the synthesis of non-natural peptides and peptidomimetics. Tetrahedron Lett. 2021, 81, 153342. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kumamoto, T. Amidines in Organic Synthesis. In Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts; Ishikawa, T., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 49–92. [Google Scholar] [CrossRef]
- Taylor, J.E.; Bull, S.D.; Williams, J.M.J. Amidines, isothioureas, and guanidines as nucleophilic catalysts. Chem. Soc. Rev. 2012, 41, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Davis, Y.A.; Danneman, M.W.; Johnston, J.N. Chiral proton catalysis of secondary nitroalkane additions to azomethine: Synthesis of a potent GlyT1 inhibitor. Chem. Commun. 2012, 48, 5578–5580. [Google Scholar] [CrossRef] [Green Version]
- Vara, B.A.; Mayasundari, A.; Tellis, J.C.; Danneman, M.W.; Arredondo, V.; Davis, T.A.; Min, J.; Finch, K.; Guy, R.K.; Johnston, J.N. Organocatalytic, diastereo- and enantioselective synthesis of nonsymmetric cis-stilbene diamines: A platform for the preparation of single-enantiomer cis-imidazolines for protein-protein inhibition. J. Org. Chem. 2014, 79, 6913–6938. [Google Scholar] [CrossRef] [Green Version]
- Ahlemeyer, N.A.; Streff, E.V.; Muthupandi, P.; Birman, V.B. Dramatic acceleration of an acyl transfer-initiated cascade by using electron-rich amidine-based catalysts. Org. Lett. 2017, 19, 6486–6489. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Cai, T.; Shen, Q. Addition of amines to nitriles catalyzed by ytterbium amides: An efficient one-step synthesis of monosubstituted N-arylamidines. Org. Lett. 2008, 10, 445–448. [Google Scholar] [CrossRef]
- Li, W.; Xue, M.; Xu, F.; Tu, J.; Zhang, Y.; Shen, Q. Synthesis, characterization of bridged bis(amidinate) lanthanide amides and their application as catalysts for addition of amines to nitriles for monosubstituted N-arylamidines. Dalton Trans. 2012, 8252–8260. [Google Scholar] [CrossRef] [PubMed]
- Garduño, J.A.; García, J.J. Synthesis of amidines and benzoxazoles from activated nitriles with Ni(0) catalysts. ACS Catal. 2015, 5, 3470–3477. [Google Scholar] [CrossRef]
- Froehner, G.; Challis, K.; Gagnon, K.; Getman, T.D.; Luck, R.L. A re-investigation of the reactions of amines and alcohols with 6,9-bis-(acetonitrile)decaborane. Synth. React. Inorg. Met. Org. Chem. 2006, 36, 777–785. [Google Scholar] [CrossRef]
- Getman, T.D.; Luck, R.L.; Cienkus, C. 2-[(1-{[3-(dimethylazaniumyl)propyl]-methylaminoethylidene)azaniumyl]- nonahydro-closo-decaborate dimethyl sulfoxide disolvate. Acta Cryst. E 2011, 67, o1682–o1683. [Google Scholar] [CrossRef]
- Losytskyy, M.Y.; Kovalska, V.B.; Varzatskii, O.A.; Kuperman, M.V.; Potocki, S.; Gumienna-Kontecka, E.; Zhdanov, A.P.; Yarmoluk, S.M.; Voloshin, Y.Z.; Zhizhin, K.Y.; et al. An interaction of the functionalized closo-borates with albumins: The protein fluorescence quenching and calorimetry study. J. Lumin. 2016, 169, 51–60. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Zhdanov, A.P.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis of substituted derivatives of closo-decaborate anion with a peptide bond: The way towards designing biologically active boron-containing compounds. Russ. J. Inorg. Chem. 2019, 64, 1499–1506. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic addition of amino acid esters to nitrilium derivatives of closo-decaborate anion. Mendeleev Commun. 2021, 31, 201–203. [Google Scholar] [CrossRef]
- Stogniy, M.Yu.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Godovikov, I.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl amidines. J. Organomet. Chem. 2020, 909, 121111. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10-NCCH2CH2OCH2CH2C≡N-7,8-C2B9H11: Synthesis and reactions with various nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Nelyubin, A.V.; Selivanov, N.A.; Klyukin, I.N.; Bykov, A.Y.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. New method for synthesis of substituted N-borylated dipeptides based on acetonitrile derivative of the closo-dodecaborate anion. Russ. J. Inorg. Chem. 2021, 66, 1390–1395. [Google Scholar] [CrossRef]
- Makarycheva-Mikhailova, A.V.; Kukushkin, V.Y.; Nazarov, A.A.; Garnovskii, D.A.; Pombeiro, A.J.L.; Haukka, M.; Keppler, B.K.; Galanski, M. Amidines derived from Pt(IV)-mediated nitrile-amino alcohol coupling and their Zn(II)-catalyzed conversion into oxazolines. Inorg. Chem. 2003, 42, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Kalz, K.F.; Hausmann, A.; Dechert, S.; Meyer, S.; John, M.; Meyer, F. Solution chemistry of N,N’-disubstituted amidines: Identification of isomers and evidence for linear dimer formation. Chem. Eur. J. 2016, 22, 18190–18196. [Google Scholar] [CrossRef]
- Belluco, U.; Benetollo, F.; Bertani, R.; Bombieri, G.; Michelin, R.A.; Mozzon, M.; Pombeiro, A.J.L.; Guedes da Silva, F.C. Stereochemical investigation of the addition of primary and secondary aliphatic amines to the nitrile complexes cis- and trans-[PtCl2(NCMe)2]. X-ray structures of the amidine complexes trans-[Pt(NH2Pri)2{Z-N(H)=C(NHPri)Me}]Cl2·4H2O and trans-[PtCl2(NCMe){E-N(H)=C(NMeBut)Me}]. Inorg. Chim. Acta 2002, 330, 229–239. [Google Scholar] [CrossRef]
- Belluco, U.; Benetollo, F.; Bertani, R.; Bombieri, G.; Michelin, R.A.; Mozzon, M.; Tonon, O.; Pombeiro, A.J.L.; Guedes da Silva, F.C. Addition reactions of primary and secondary aliphatic amines to the benzonitrile ligands in cis- and trans-[PtCl2(NCPh)2] complexes. X-ray structure of the amidine complex trans-[PtCl2{Z-N(H)=C(NHBut)Ph}2]. Inorg. Chim. Acta 2002, 334, 437–447. [Google Scholar] [CrossRef]
- Maresca, L.; Natile, G.; Intini, F.P.; Gasparrini, F.; Tiripiccbio, A.; Tiripicchio-Camellinil, M. Nucleophilic attack of amine and hydroxide to platinum dibenzonitrile dichloride. Crystal structure of [Pt(NH=CPhN-t-BuCH2CH2NH-t- Bu)Cl(NHCO(Ph)] (2) and cis-[Pt(NH=CPhN-t-BuCH2CH2NH-t-Bu)Cl2(NCPh)] (3). J. Am. Chem. Soc. 1986, 108, 1180–1185. [Google Scholar] [CrossRef]
- Michelin, R.A.; Betani, R.; Mozzon, M.; Sassi, A.; Benetollo, F.; Bombieri, G.; Pombeiro, A.J.L. cis-Addition of dimethylamine to the coordinated nitriles of cis- and trans-[PtCl2(NCMe)2]. X-ray structure of the amidine complex cis- [PtCl2{E-N(H)=C(NMe2)Me}2]·CH2Cl2]. Inorg. Chem. Commun. 2001, 4, 275–280. [Google Scholar] [CrossRef]
- Bacchi, A.; Belli Dell’ Amico, D.; Calderazzo, F.; Labella, L.; Pelizzi, G.; Marchetti, F.; Samaritani, S. Reactions of the homoleptic acetonitrile complexes of palladium and platinum with diethylamine. Inorg. Chim. Acta 2010, 363, 2467–2473. [Google Scholar] [CrossRef]
- Chin, C.S.; Chong, D.; Lee, B.; Jeong, H.; Won, G.; Do, Y.; Park, Y.J. Activation of acetonitrile in [Cp*Ir(η3- CH2CHCHPh)(NCMe)]+: Crystal structures of iridium-amidine, imino-ether, amido, and amide complexes. Organometallics 2000, 19, 638–648. [Google Scholar] [CrossRef]
- Podjed, N.; Modec, B.; Alcaide, M.; Lopez-Serrano, J. From cyclic amines and acetonitrile to amidine zinc(II) complexes. RSC Adv. 2020, 10, 18200–18221. [Google Scholar] [CrossRef]
- Richardson, T.; de Gala, S.; Crabtree, R.H.; Siegbahn, P.E.M. Unconventional hydrogen bonds: Intermolecular B-H···H-N interactions. J. Am. Chem. Soc. 1995, 117, 12875–12876. [Google Scholar] [CrossRef]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the N-H···H-B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.-C.; Shore, S.G. The roles of dihydrogen bonds in amine borane chemistry. Acc. Chem. Res. 2013, 46, 2666–2675. [Google Scholar] [CrossRef]
- Huang, Z.; Lingam, H.K.; Chen, X.; Porter, S.; Du, A.; Woodard, P.M.; Shore, S.G.; Zhao, J.-C. Synthesis, structural analysis, and thermal decomposition studies of [(NH3)2BH2]B3H8. RSC Adv. 2013, 3, 7460–7465. [Google Scholar] [CrossRef]
- Tan, Y.; Gu, Q.; Kimpton, J.A.; Li, Q.; Chen, X.; Ouyang, L.; Zhu, M.; Sun, D.; Yu, X. A synergistic strategy established by the combination of two H-enriched B-N based hydrides towards superior dehydrogenation. J. Mater. Chem. A 2013, 1, 10155–10165. [Google Scholar] [CrossRef]
- Guan, R.; Wang, P.; Song, Y.; Staroverov, V.N. Pressure-induced polymorphic transformations of ethylenediamine bisborane. J. Phys. Chem. C 2021, 125, 18614–18622. [Google Scholar] [CrossRef]
- Orlova, A.M.; Mustyatsa, V.N.; Goeva, L.V.; Katser, S.B.; Solntsev, K.A.; Kuznetsov, N.T. Bipyridylammonium salts of closo-borate anions BnHn2- (n = 6, 10, and 12). Russ. J. Inorg. Chem. 1996, 41, 1856–1862. [Google Scholar]
- Mebs, S.; Kalinowski, R.; Grabowsky, S.; Förster, D.; Kickbusch, R.; Justus, E.; Morgenroth, W.; Paulmann, C.; Luger, P.; Gabel, D.; et al. Charge transfer via the dative N-B bond and dihydrogen contacts. Experimental and theoretical electron density studies of four deltahedral boranes. J. Phys. Chem. A 2011, 115, 1385–1395. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Malinina, E.A.; Kuznetsov, N.T. Dihydrogen bonds in salts of boron cluster anions [BnHn]2- with protonated heterocyclic organic bases. Crystals 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Schulz, F.; Sumerin, V.; Heikkinen, S.; Pedersen, B.; Wang, C.; Atsumi, M.; Leskela, M.; Repo, T.; Pyykko, P.; Petry, W.; et al. Molecular hydrogen tweezers: Structure and mechanisms by neutron diffraction, NMR, and deuterium labeling studies in solid and solution. J. Am. Chem. Soc. 2011, 133, 20245–20257. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Rit, A.; Campos, J.; Kolychev, E.L.; Aldridge, S. Catalytic B-N dehydrogenation using frustrated Lewis pairs: Evidence for a chain-growth coupling mechanism. J. Am. Chem. Soc. 2016, 138, 3306–3309. [Google Scholar] [CrossRef] [Green Version]
- Zhdanov, A.P.; Voinova, V.V.; Klyukin, I.N.; Kubasov, A.S.; Zhizhin, K.Y.; Kuznetsov, N.T. New synthesis method of N-monosubstituted ammonium-closo-decaborates. J. Cluster Sci. 2019, 30, 1327–1333. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Burlington, VT, USA, 2009. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, E.V.; Stogniy, M.Y.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide). Molecules 2021, 26, 6544. https://doi.org/10.3390/molecules26216544
Bogdanova EV, Stogniy MY, Suponitsky KY, Sivaev IB, Bregadze VI. Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide). Molecules. 2021; 26(21):6544. https://doi.org/10.3390/molecules26216544
Chicago/Turabian StyleBogdanova, Ekaterina V., Marina Yu. Stogniy, Kyrill Yu. Suponitsky, Igor B. Sivaev, and Vladimir I. Bregadze. 2021. "Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide)" Molecules 26, no. 21: 6544. https://doi.org/10.3390/molecules26216544
APA StyleBogdanova, E. V., Stogniy, M. Y., Suponitsky, K. Y., Sivaev, I. B., & Bregadze, V. I. (2021). Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide). Molecules, 26(21), 6544. https://doi.org/10.3390/molecules26216544