Thymodepressin—Unforeseen Immunosuppressor
Abstract
:1. Introduction
1.1. Historical Background
1.2. Discovery of Thymodepressin
1.3. Thymodepressin as an Immunosuppressor. Biological Activity and Medical Applications
- -
- Thymodepressin inhibits the proliferation of PHA-stimulated human lymphocytes, as well as the spontaneous proliferation of human thymus cells [51].
- -
- Thymodepressin inhibits migration of CD34+ cells from the bone marrow into peripheral blood both in normal and tumor-bearing animals [50].
- -
- Prophylactic application of Thymodepressin leads to earlier restoration of the population of both committed (CFU-C-8) and pluripotent (CFU-C-12) hematopoietic precursors after exposure to the cytostatic cytosine arabinoside (cytosar) [52].
- -
- Thymodepressin injected into donor mice two days before irradiation at a dose of 4 Gy promotes more intensive restoration of the population of hematopoietic progenitor cells than the control group [53].
- -
- Thymodepressin suppresses the development of graft-versus-host-reaction after allogenic bone marrow transplantation in mice [42].
2. Results and Discussion
2.1. Comparison of Thymodepressin and Cyclosporin A in an Experimental Autoimmunity Model
2.1.1. Development of Anti-Fibrillarin Antibodies (AFA) in Control Groups
2.1.2. The Effect of Cyclosporin A in the Prophylactic Regimen
2.1.3. The Effect of Thymodepressin in the Prophylactic Regimen
2.1.4. Comparison of the Effects of Thymodepressin and Cyclosporin A in the Prophylactic Regimen
2.1.5. Effect of Cyclosporin A in the Therapeutic Regimen
2.1.6. Effect of Thymodepressin in the Therapeutic Regimen
2.1.7. Comparison of the Effects of Thymodepressin and Cyclosporin A in a Therapeutic Regimen
2.1.8. Summary
2.2. Comparison of the Effects of Thymodepressin and Cyclosporine A on the Graft-Versus-Host-Reaction Developed after Allogenic Spleen Cells Transplantation
3. Conclusions and Prospects
4. Materials and Methods
4.1. Reagents
- mercury chloride (HgCl2) (Sigma, Burlington, MA, USA);
- sodium chloride (NaCl) (JSC “Biochemist”, Moscow, Russia);
- Sandimmun® (Novartis Pharma AG, Basel, Switzerland);
- Thymodepressin® (Peptos Pharma, Moscow, Russia);
- ethanol 96%;
- Phosphate-buffered saline PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.2–7.4);
- HEp-2 cell preparations (Bio-Rad Laboratories, Hercules, CA, USA);
- Moviol (Sigma, Ronkonkoma, NY, USA);
- DABCO (1,4-Diazobicyclo [2.2.2] octane) (Sigma, Ronkonkoma, NY, USA);
- Antibodies:
- -
- rabbit polyclonal antibodies to fibrillarin (Abcam, Boston, MA, USA);
- -
- goat antibodies to mouse immunoglobulins conjugated to fluorescein isothiocyanate (IMTEK, Moscow, Russia);
- -
- goat antibodies to rabbit immunoglobulins conjugated with tetrarodamine isothiocyanate (SouthernBiotech Associates, Birmingham, AL, USA);
- -
- goat antibodies to human immunoglobulins conjugated with tetrarodamine isothiocyanate (Santa Cruz Biotechnology, Dallas, TX, USA);
- -
- antibodies to mouse immunoglobulins conjugated with horseradish peroxidase (Sigma, Ronkonkoma, NY, USA).
4.2. Equipment
- thermostat “Biological Thermostat BT 120” (Labsystems, East Yorkshire, UK);
- centrifuge “Eppendorf 5804 R” (Eppendorf, Hamburg, Germany);
- MiniSpin Plus centrifuge (Eppendorf, Hamburg, Germany);
- Beckman J2-21 centrifuge (Beckman, Indianapolis, IN, USA);
- fluorescent microscope Axiovert 200 (Carl Zeiss, Göttingen, Germany);
- 13-bit monochrome digital camera CoolSnapcf (Roper Scientific, Sarasota, FL, USA);
- Axioscop A1 microscope (Carl Zeiss, Germany);
- digital color camera AxioCam MRc5 (Carl Zeiss, Germany);
- cryotome (MICRO, Walldorf, Germany);
- pH meter MP220 (Mettler Toledo, Columbus, OH, USA);
- electrophoretic chamber Mighty Small II (AmershamPharmaciaBiotech, Piscataway, NJ, USA);
- chamber for semi-dry electroblotting (BioRad, Hercules, CA, USA); PowerPac HC power supply (BioRad);
- EPS 601 power supply (AmershamParmaciaBiotech, USA);
- Direct-Q 5 tap water purification system (Millipore, Burlington, MA, USA);
- automatic pipettes of various calibrations (Eppendorf, Framingham, MA, USA);
- plastic tubes of 1.5 mL (Eppendorf, Germany).
4.3. Methods
4.3.1. Detection of Autoantibodies to Fibrillarin
Indirect Immunofluorescence Method
Western Blotting
Colocalization of an Autoantigen Recognized by the Sera of Autoimmune Mice with FiBrillarin
4.3.2. Identification of Immune Complexes in the Kidneys
4.4. Mice
4.5. Substances Used for Treatment
4.6. The Induction of an Autoimmune Process
4.7. Blood Sampling
4.8. Autoantibody Detection
4.9. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Sample Availability
References
- Goldstein, A. Thymic Hormones and Lymphokines; Plenum Press: New York, NY, USA, 1984; pp. 37–42. [Google Scholar]
- Goldstein, A.L. History of the discovery of the thymosins. Ann. N. Y. Acad. Sci. 2007, 1112, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, P.; Mazzanti, P.; Kouttab, N.M. Update and future perspectives of a thymic biological response modifier (thymomodulin). Immunopharm. Immunotoxicol. 1987, 9, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Kouttab, N.M.; Prada, M.; Cazzola, P. Thymomodulin: Biological properties and clinical applications. Med. Oncol. Tumor Pharmacother. 1989, 6, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Safieh-Garabedian, B.; Kendall, M.D.; Khamashta, M.A.; Hughes, G.R. Thymulin and its role in immunomodulation. J. Autoimmune 1992, 5, 547–555. [Google Scholar] [CrossRef]
- Arion, V.Y.; Zimina, I.V.; Moskvina, S.N.; Bystrova, O.V. Tactivin—Natural immunocorrector. Clinical application. Immunopathol. Allergol. Infectol. 2007, 4, 11–26. [Google Scholar]
- Garaci, E. Thymosin alpha1: A historical overview. Ann. N. Y. Acad. Sci. 2007, 1112, 14–20. [Google Scholar] [CrossRef]
- Low, T.L.; Hu, S.K.; Goldstein, A.L. Complete amino acid sequence of bovine thymosin beta 4: A thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc. Natl. Acad. Sci. USA 1981, 78, 1162–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audhya, T.; Schlesinger, D.H.; Goldstein, G. Complete amino acid sequences of bovine thymopoietins I, II, and III: Closely homologous polypeptides. Biochemistry 1981, 20, 6195–6200. [Google Scholar] [CrossRef]
- Goldstein, G.; Scheid, M.P.; Boyse, E.A.; Schlesinger, D.H.; Van Wauwe, J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science 1979, 204, 1309–1310. [Google Scholar] [CrossRef]
- Audhya, T.; Scheid, M.P.; Goldstein, G. Contrasting biological activities of thymopoietin and splenin, two closely related polypeptide products of thymus and spleen. Proc. Natl. Acad. Sci. USA 1984, 81, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Audhya, T.; Schlesinger, D.H.; Goldstein, G. Isolation and complete amino acid sequence of human thymopoietin and splenin. Proc. Natl. Acad. Sci. USA 1987, 84, 3545–3549. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Biswas, S.; Mathur, K.B.; Haq, W.; Garg, S.K.; Agarwal, S.S. Thymopentin and splenopentin as immunomodulators. Immunol. Res. 1998, 17, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Clumeck, N.; Cran, S.; Van de Perre, P.; Mascart-Lemone, F.; Duchateau, J.; Bolla, K. Thymopentin treatment in AIDS and pre-AIDS patients. Surv. Immunol. Res. 1985, 4, 58–62. [Google Scholar] [PubMed]
- Dardenne, M.; Pleau, J.M.; Man, N.K.; Bach, J.F. Structural study of circulating thymic factor: A peptide isolated from pig serum. I. Isolation and purification. J. Biol. Chem. 1977, 252, 8040–8044. [Google Scholar] [CrossRef]
- Bach, J.; Bardenne, M.; Pleau, J.; Rosa, J. Biochemical characterisation of a serum thymic factor. Nature 1977, 266, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, M.; Pléau, J.M.; Blouquit, J.Y.; Bach, J.F. Characterization of facteur thymique sérique (FTS) in the thymus. II. Direct demonstration of the presence of FTS in thymosin fraction V. Clin. Exp. Immunol. 1980, 42, 477–482. [Google Scholar]
- Cordero, O.J.; Maurer, H.R.; Nogueira, M. Novel approaches to immunotherapy using thymic peptides. Immunol. Today 1997, 18, 10–13. [Google Scholar] [CrossRef]
- Petrov, R.V.; Mikhajlova, A.A.; Stepanenko, R.N.; Zakharova, L.A. Cell interactions in the immune response: Effect of humoral factor released from bone marrow cells on the quantity of mature antibody producers in culture of immune lymph node cells. Cell Immunol. 1975, 17, 342–350. [Google Scholar] [CrossRef]
- Petrov, R.V.; Mikhailova, A.A.; Zakharova, L.A.; Sergeyev, J.O.; Novicov, V.I. Cell interactions at the level of mature antibody producers: The properties of the bone marrow humoral factor stimulating antibody production. Ann. Immunol. 1980, 131D, 161–171. [Google Scholar]
- Petrov, R.V.; Mikhailova, A.A.; Zakharova, L.A.; Vasilenko, A.M.; Katlinsky, A.V. Myelopeptides—Bone marrow mediators with immunostimulating and endorphin-like activity. Scand. J. Immunol. 1986, 24, 237–243. [Google Scholar] [CrossRef]
- Mikhailova, A.A. Myelopeptides and Immunoreabilitation. Int. J. Immunoreabil. 1997, 5, 5. [Google Scholar]
- Petrov, R.V.; Mikhailova, A.A.; Fonina, L.A. Bone marrow immunoregulatory peptides (myelopeptides): Isolation, structure, and functional activity. Biopolymers 1997, 43, 139–146. [Google Scholar] [CrossRef]
- Fonina, L.A.; Kudriavtseva, E.V.; Bespalova, Z.D.; Efremov, M.A.; Mikhaĭlova, A.A.; Kirilina, E.A. Synthesis and properties of the myelopeptides with differentiating activity. Bioorg. Khim. 2010, 36, 493–497. [Google Scholar] [CrossRef]
- Morozov, V.G.; Khavinson, V.K. Natural and synthetic thymic peptides as therapeutics for immune dysfunction. Int. J. Immunopharmacol. 1997, 19, 501–505. [Google Scholar] [CrossRef]
- Khavinson, V.K. Peptide medicines: Past, present, future. Klin. Meditsina (Clin. Med. Russ.) 2020, 98, 165–177. [Google Scholar] [CrossRef]
- Kuznik, B.I.; Khavinson, V.K. The effect of Thymalin on the immune system, hemostasis and the level of cytokines in patients with various diseases. Prospects for use in COVID-19. Russ. J. Vrach. 2020, 31, 18–26. [Google Scholar] [CrossRef]
- Khavinson, V.K. Peptides and Ageing. Neurol. Endocrinol. Lett. 2002, 23, 11–144. [Google Scholar]
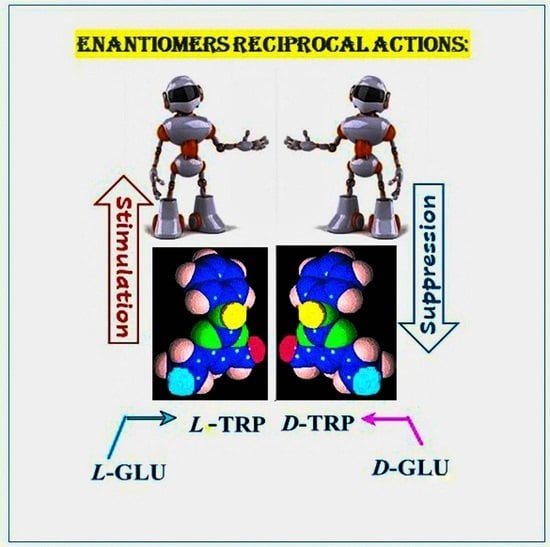
- Deigin, V.I.; Poverenny, A.M.; Semina, O.V.; Semenets, T.N. Reciprocal effect of optical isomerism of EW-dipeptides on immune response. Immunol. Lett. 1999, 67, 41–46. [Google Scholar] [CrossRef]
- Deigin, V.I.; Poverenny, A.M.; Semina, O.V.; Semenets, T.N. Stimulation and suppression of the immune response and hemopoiesis by novel natural and synthetic peptides. In Peptides for the New Millennium. American Peptide Symposia; Springer: Berlin/Heidelberg, Germany, 2000; p. 741. [Google Scholar]
- Reggiani, P.C.; Morel, G.R.; Cónsole, G.M.; Barbeito, C.G.; Rodriguez, S.S.; Brown, O.A.; Bellini, M.J.; Pléau, J.M.; Dardenne, M.; Goya, R.G. The thymus-neuroendocrine axis: Physiology, molecular biology, and therapeutic potential of the thymic peptide thymulin. Ann. N. Y. Acad. Sci. 2009, 1153, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Haddad, J.J.; Saade, N.E. Thymulin: An emerging anti-inflammatory molecule. Curr. Med. Chem. 2005, 4, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.X.; Wan, W.L.; Li, H.S.; Wang, J.; Chen, G.A.; Ke, X.Y. Thymopentin enhances the generation of T-cell lineage derived from human embryonic stem cells in vitro. Exp. Cell Res. 2015, 331, 387–398. [Google Scholar] [CrossRef]
- Ashapkin, V.; Khavinson, V.; Shilovsky, G.; Linkova, N.; Vanuyshin, B. Gene expression in human mesenchymal stem cell aging cultures: Modulation by short peptides. Mol. Biol. Rep. 2020, 47, 4323–4329. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.I.; Semenets, T.N.; Zamulaeva, I.A.; Maliutina, Y.V.; Selivanova, E.I.; Saenko, A.S.; Semina, O.V. The effects of the EW dipeptide optical and chemical isomers on the CFU-S population in intact and irradiated mice. Int. Immunopharmacol. 2007, 7, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Semina, O.V.; Semenets, T.N.; Zamulaeva, I.A.; Selivanova, E.I.; Iljina, T.P.; Maliutina, Y.V.; Semin, D.Y.; Deigin, V.I.; Saenko, A.S. Dipeptide gamma-d-Glu-d-Trp (thymodepressin) inhibits migration of CD34+ cells from the bone marrow into peripheral blood during tumor growth. Bull. Exp. Biol. Med. 2008, 146, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.; Ksenofontova, O.; Khrushchev, A.; Yatskin, O.; Goryacheva, A.; Ivanov, V. Chemical Platform for the Preparation of Synthetic Orally Active Peptidomimetics with Hemoregulating Activity. ChemMedChem 2016, 11, 1974–1977. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef] [Green Version]
- Shemyakin, M.M.; Ovchinnikov, Y.A.; Ivanov, V.T.; Evstratov, A.V. Topochemical approach in studies of the structure-activity relation: Enantio-enniatin B. Nature 1967, 213, 412–413. [Google Scholar] [CrossRef]
- Shemyakin, M.M.; Ovchinnikov, Y.A.; Ivanov, V.T.; Antonov, V.K.; Vinogradova, E.I.; Shkrob, A.M.; Malenkov, G.G.; Evstratov, A.V.; Laine, I.A.; Melnik, E.I.; et al. Cyclodepsipeptides as chemical tools for studying ionic transport through membranes. J. Membr. Biol. 1969, 1, 402–430. [Google Scholar] [CrossRef]
- Vinogradova, I.E.; Zamulaeva, I.A.; Pavlov, V.V.; Selivanova, E.I.; Deĭgin, V.I.; Smirnova, S.G.; Orlova, N.V.; Saenko, A.S. Application ot thymodepressin for treating autoimmune cyclopenia. Ter. Arkh. 2002, 74, 64–67. [Google Scholar]
- Poverenny, A.M.; Semina, O.V.; Semenets, T.N.; Zamulaeva, I.A.; Selivanova, E.I.; Deigin, V.I. Thymodepressin, inhibiting the development of the graft-versus-host reaction. Immunology 2002, 2, 102–104. [Google Scholar]
- Sapuntsova, S.G.; Mel’nikova, N.P.; Deigin, V.I.; Kozulin, E.A.; Timoshin, S.S. Proliferative processes in the epidermis of patients with atopic dermatitis treated with thymodepressin. Bull. Exp. Biol. Med. 2002, 133, 488–490. [Google Scholar] [CrossRef]
- Blair, H.A. Etelcalcetide: First Global Approval. Drugs 2016, 76, 787–1792. [Google Scholar] [CrossRef] [PubMed]
- Poverennyĭ, A.M.; Vinogradova, I.E.; Deĭgin, V.I. Gemoreguliatornye sinteticheskie peptidy. Ter. Arkh. 2000, 72, 74–76. [Google Scholar]
- Vladimirskaya, E.B.; Osipova, Y.E.; Kaznacheev, K.S.; Ivanova, K.A.; Deigin, V.I.; Rumyantsev, A.G. The effect of Thymodepressin on the proliferation of human hematopoietic progenitor cells. Hematol. Transfusiol. 1999, 44, 11–14. [Google Scholar]
- Vladimirskaya, E.B.; Ivanova, A.A.; Deigin, V.I.; Osipova, Y.E.; Zakharov, M.V. Influence of thymic peptides of Thymodepressin and Neogen on the proliferation of hematopoietic progenitor cells. Hematol. Transfusiol. 2000, 45, 6–8. [Google Scholar]
- Till, J.E.; McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Becker, A.J.; McCulloch, E.A.; Siminovitch, L.; Till, J.E. The effect of differing demands for blood cell production on DNA synthesis by hemopoietic colony-forming cells of mice. Blood 1965, 26, 296–308. [Google Scholar] [CrossRef]
- Vinogradova, I.E.; Vinogradov, D.L.; Poverennyĭ, A.M.; Tsyb, A.F. Autoimmunnye tireoidity u bol’nykh nekotorymi gematologicheskimi zabolevaniiami. Ter. Arkh. 1994, 66, 65–68. [Google Scholar]
- Dambaeva, S.V.; Kim, K.F.; Mazurov, D.V.; Deĭgin, V.I. Vliianie timicheskikh peptidov na funktsional’nuiu aktivnost’ fagotsitarnykh kletok perifericheskoĭ krovi donorov. Zh. Mikrobiol. Epidemiol. Immunobiol. 2002, 6, 55–59. [Google Scholar]
- Semenets, T.N.; Semina, O.V.; Vinogradova, Y.E.; Deigin, V.I.; Poverenny, A.M. Use of synthetic immunomodulating peptides to restore hematopoiesis in mice after cytostatic cytosine-arabinoside (Ara-C). Immunology 2000, 6, 20–22. [Google Scholar]
- Semina, O.V.; Semenets, T.N.; Zamulaeva, I.A.; Selivanova, E.I.; Malyutina, Y.V.; Semin, Y.A.; Deigin, V.I.; Saenko, A.S. Effects of iEW synthetic peptide isomers on bone marrow colony-forming capacity in vivo. Bull. Exp. Biol. Med. 2005, 140, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, J.E.; Deigin, V.I.; Korotkov, A.M.; Semina, O.V.; Sements, A.M.; Poverenny, T.N. The use of Thymogen for the treatment of patients with diseases of the blood system. 1. The effect of Thymogen on the granulocytic lineage of hematopoiesis in patients with depression of hematopoiesis. Russ. J. Oncol. 1999, 2, 45–48. [Google Scholar]
- Dyadkin, V.Y.; Shamov, B.A. The experimental application of Thymodepressin in patients with psoriasis. Dermatology 2003, 1, 36. [Google Scholar]
- Isaeva, T.A. Thymodepressin in psoriasis treatment. Dermatology 2003, 1, 44. [Google Scholar]
- Vinogradov, D.L.; Vinogradova, Y.E. Churg-Strauss Syndrome Accompanied by Autoimmune Thrombocytopenia. 20 years of Experience. Arch. Inner Med. 2015, 4, 69–72. [Google Scholar]
- Deigin, V.; Ksenofontova, O.; Yatskin, O.; Goryacheva, A.; Ignatova, A.; Feofanov, A.; Ivanov, V. Novel platform for the preparation of synthetic orally active peptidomimetics with hemoregulating activity. II. Hemosuppressor activity of 2,5-diketopiperazine-based cyclopeptides. Int. Immunopharmacol. 2020, 81, 106185. [Google Scholar] [CrossRef]
- Forsythe, P.; Paterson, S. Ciclosporin 10 years on: Indications and efficacy. Vet. Rec. 2014, 174, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar]
- Reuter, R.; Tessars, G.; Vohr, H.W.; Gleichmann, E.; Lührmann, R. Mercuric chloride induces autoantibodies against U3 small nuclear ribonucleoprotein in susceptible mice. Proc. Natl. Acad. Sci. USA 1989, 86, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.B.; Hultman, P. Mercury-induced autoimmunity in mice. Environ. Health Perspect. 2002, 110, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Krasilshchikova, M.; Leonov, V.; Zatsepina, O.; Deigin, V. Immunosuppressor studies of Thymodepressin in the experimental autoimmune model. Immunology 2009, 5, 290–294. [Google Scholar]
- Kapturczak, M.H.; Meier-Kriesche, H.U.; Kaplan, B. Pharmacology of calcineurin antagonists. Transpl. Proc. 2004, 36, 25S–32S. [Google Scholar] [CrossRef]
- Ponticelli, C.; Glassock, R.J. Prevention of complications from use of conventional immunosuppressants: A critical review. J. Nephrol. 2019, 32, 851–870. [Google Scholar] [CrossRef]
- Mihatsch, M.J.; Kyo, M.; Morozumi, K.; Yamaguchi, Y.; Nickeleit, V.; Ryffel, B. The side-effects of ciclosporine-A and tacrolimus. Clin. Nephrol. 1998, 49, 356–363. [Google Scholar] [PubMed]
- Bundick, R.V.; Craggs, R.I.; Holness, E. The effect of cyclosporin A, FK506, and rapamycin on the murine chronic graft-versus-host response—An in vivo model of Th2-like activity. Clin. Exp. Immunol. 1995, 99, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V. Multifunctional Bioactive Compounds. U.S. Patent US8637521B2, 4 August 2006. [Google Scholar]
- Hansson, M.; Abedi-Valugerdi, M. Xenobiotic metal-induced autoimmunity: Mercury and silver differentially induce antinucleolar autoantibody production in susceptible H-2s, H-2q and H-2f mice. Clin. Exp. Immunol. 2003, 131, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, K.; Hultman, P. The role of Fc-receptors in murine mercury-induced systemic autoimmunity. Clin. Exp. Immunol. 2006, 144, 309–318. [Google Scholar] [CrossRef]









| Groups | Regimens of HgCl2 Administration | Time (in Weeks) | ||
|---|---|---|---|---|
| 4th | 6th | 8th | ||
| 1 | HgCl2 only | 800 ± 331 | 1500 ± 214 | 1071 ± 333 |
| HgCl2 simultaneously with: | ||||
| 2 | Thymodepressin 0.14 mg/kg | 250 ± 137 | 406 ± 219 | 313 ± 180 |
| 3 | Thymodepressin 0.35 mg/kg | 486 ± 349 | 379 ± 337 | 307 ± 201 |
| 4 | Thymodepressin 0.7 mg/kg | 108 ± 155 | 208 ± 153 | 142 ± 144 |
| 5 | Cyclosporin A 20 mg/kg | 180 ± 282 | 500 ± 346 | 1200 ± 733 |
| 6 | Cyclosporin A 50 mg/kg | 10 ± 20 | 150 ± 294 | 160 ± 290 |
| Groups | Regimens of HgCl2 Administration | Time (in Weeks) | |||
|---|---|---|---|---|---|
| 6th | 8th | 10th | 13th | ||
| 1 | HgCl2 only | 800 ± 331 | 1500 ± 214 | 1071 ± 333 | 667 ± 175 |
| 2 | Thymodepressin 0.14 mg/kg | 775 ± 481 | 383 ± 270 | 433 ± 284 | 294 ± 14 |
| 3 | Thymodepressin 0.35 mg/kg | 450 ± 213 | 450 ± 227 | 325 ± 137 | 258 ± 179 |
| 4 | Thymodepressin 0.7 mg/kg | 1079 ± 288 | 550 ± 185 | 443 ± 212 | 452 ± 135 |
| 5 | Cyclosporin A 20 mg/kg | 1620 ± 1446 | 1470 ± 1099 | 1720 ± 1188 | 1720 ± 1343 |
| 6 | Cyclosporin A 50 mg/kg | 1250 ± 620 | 1000 ± 438 | 650 ± 427 | 810 ± 635 |
| 7 | Cyclosporin A 125 mg/kg | 3125 ± 1715 | 2033 ± 1641 | 1367 ± 1455 | 1700 ± 1444 |
| Group | Mice # | Splenomegaly Index | % Suppression |
|---|---|---|---|
| Control | 15 | 10.2 ± 0.8 * | - |
| Cyclosporine A | 15 | 8.8 ± 0.5 | 34.6 |
| Thymodepressin | 15 | 7.3 ± 1.0 * | 70.7 |
| Intact | 10 | 6.1 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deigin, V.I.; Vinogradova, Y.E.; Vinogradov, D.L.; Krasilshchikova, M.S.; Ivanov, V.T. Thymodepressin—Unforeseen Immunosuppressor. Molecules 2021, 26, 6550. https://doi.org/10.3390/molecules26216550
Deigin VI, Vinogradova YE, Vinogradov DL, Krasilshchikova MS, Ivanov VT. Thymodepressin—Unforeseen Immunosuppressor. Molecules. 2021; 26(21):6550. https://doi.org/10.3390/molecules26216550
Chicago/Turabian StyleDeigin, Vladislav I., Yulia E. Vinogradova, Dmitry L. Vinogradov, Marina S. Krasilshchikova, and Vadim T. Ivanov. 2021. "Thymodepressin—Unforeseen Immunosuppressor" Molecules 26, no. 21: 6550. https://doi.org/10.3390/molecules26216550
APA StyleDeigin, V. I., Vinogradova, Y. E., Vinogradov, D. L., Krasilshchikova, M. S., & Ivanov, V. T. (2021). Thymodepressin—Unforeseen Immunosuppressor. Molecules, 26(21), 6550. https://doi.org/10.3390/molecules26216550