Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy
Abstract
:1. Introduction
2. Pathologies Correlated with Metal Dyshomeostasis
2.1. Genetic Disorders
2.2. Acute and Chronic Metal Intoxication
2.3. Multifactorial Etiology
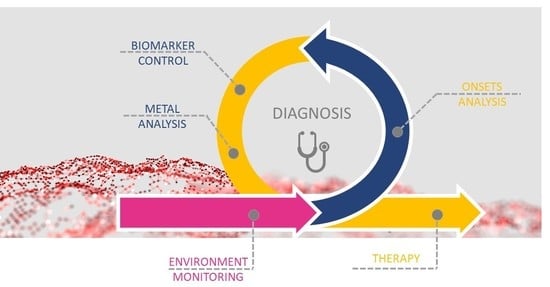
3. Exposure, Diagnosis, and Therapy of Metal-Related Diseases
3.1. Environmental Monitoring
3.2. Metal Accumulation Sites/Tissues
3.3. Drugs Enhancing/Involved in Metal Dyshomeostasis
3.4. Diagnosis
3.5. Therapy for Metal-Related Pathologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is there evidence for man-made nanoparticles in the Dutch environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M.; Medici, S.; Zoroddu, M.A. Toxicity of nanoparticles: Etiology and mechanisms. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–546. [Google Scholar]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef] [Green Version]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef]
- Park, J.H.; Hogrebe, M.; Fobker, M.; Brackmann, R.; Fiedler, B.; Reunert, J.; Rust, S.; Tsiakas, K.; Santer, R.; Grüneberg, M. SLC39A8 deficiency: Biochemical correction and major clinical improvement by manganese therapy. Genet. Med. 2018, 20, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piperno, A.; Alessio, M. Aceruloplasminemia: Waiting for an efficient therapy. Front. Neurosci. 2018, 12, 903. [Google Scholar] [CrossRef] [Green Version]
- Lehn, A.; Boyle, R.; Brown, H.; Airey, C.; Mellick, G. Neuroferritinopathy. Parkinsonism Relat. Disord. 2012, 18, 909–915. [Google Scholar] [CrossRef]
- Shribman, S.; Reid, E.; Crosby, A.H.; Houlden, H.; Warner, T.T. Hereditary spastic paraplegia: From diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019, 18, 1136–1146. [Google Scholar] [CrossRef]
- Margetis, K.; Korfias, S.; Boutos, N.; Gatzonis, S.; Themistocleous, M.; Siatouni, A.; Dalivigka, Z.; Flaskas, T.; Stranjalis, G.; Boviatsis, E. Intrathecal baclofen therapy for the symptomatic treatment of hereditary spastic paraplegia. Clin. Neurol. Neurosurg. 2014, 123, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Abusrair, A.H.; Bohlega, S.; Al-Semari, A.; Al-Ajlan, F.S.; Al-Ahmadi, K.; Mohamed, B.; AlDakheel, A. Brain MR Imaging Findings in Woodhouse-Sakati Syndrome. Am. J. Neuroradiol. 2018, 39, 2256–2262. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.A.; Hardy, J.; Bhatia, K.P. Syndromes of neurodegeneration with brain iron accumulation (NBIA): An update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov. Disord. 2012, 27, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Veneri, D. Recent advances in hereditary hemochromatosis. Ann. Hematol. 2005, 84, 347–352. [Google Scholar] [CrossRef]
- Brewer, G.J.; Askari, F.K. Wilson’s disease: Clinical management and therapy. J. Hepatol. 2005, 42, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Travaglini, L.; Drouin, C.A.; Ceballos-Picot, I.; Rizza, T.; Bertini, E.; Carrozzo, R.; Petrini, S.; De Lonlay, P.; El Hachem, M. MEDNIK syndrome: A novel defect of copper metabolism treatable by zinc acetate therapy. Brain 2013, 136, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, B.; Lingertat-Walsh, K.; Clarke, J.T. Copper-histidine therapy for Menkes disease. J. Pediatr. 1993, 123, 828–830. [Google Scholar] [CrossRef]
- Ogawa, E.; Kodama, H. Effects of disulfiram treatment in patients with Menkes disease and occipital horn syndrome. J. Trace Elem. Med. Biol. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Bindu, P.S.; Chiplunkar, S.; Vandana, V.; Nagappa, M.; Govindaraj, P.; Taly, A. Huppke-Brendel Syndrome—GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Puri, N.; Puri, A. A study on efficacy of oral zinc therapy for treatment of acrodermatitis enteropathica. Our Dermatol. Online 2013, 4, 162. [Google Scholar] [CrossRef] [Green Version]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and skin: An update. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Mantle, D.; Wilkins, R.; Preedy, V. A novel therapeutic strategy for Ehlers–Danlos syndrome based on nutritional supplements. Med. Hypotheses 2005, 64, 279–283. [Google Scholar] [CrossRef]
- Rossi, M.; Balint, B.; Millar Vernetti, P.; Bhatia, K.P.; Merello, M. Genetic dystonia-ataxia syndromes: Clinical spectrum, diagnostic approach, and treatment options. Mov. Disord. Clin. Pract. 2018, 5, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-h.; Wang, X.-f.; Yang, G.; Wei, J.; Tan, W.-h.; Wang, L.-x.; Guo, X.; Lammi, M.J.; Xu, J.-H. Efficacy of long-term selenium supplementation in the treatment of chronic Keshan disease with congestive heart failure. Curr. Med. Sci. 2019, 39, 237–242. [Google Scholar] [CrossRef]
- Maani, N.; Karolczak, S.; Dowling, J.J. Genetic therapy for congenital myopathies. Curr. Opin. Neurol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Alfadhel, M. Genetic Disorders Associated with Metal Metabolism. Cells 2019, 8, 1598. [Google Scholar] [CrossRef] [Green Version]
- Aaseth, J.; Crisponi, G.; Anderson, O. Chelation Therapy in the Treatment of Metal Intoxication; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity induced by low thallium doses in living hippocampal neurons: Evidence of early onset mitochondrial dysfunction and correlation with ethanol production. ACS Chem. Neurosci. 2018, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, G. Thallium in the environment and health effects. Environ. Geochem. Health 2000, 22, 275–280. [Google Scholar] [CrossRef]
- Snipes, G.M.; Hafeez, A.; Marek, G.; Winchester, D.E. Sinus bradycardia with haemodynamic compromise following lithium intoxication. BMJ Case Rep. CP 2021, 14, e242946. [Google Scholar] [CrossRef]
- Diserens, L.; Porretta, A.P.; Trana, C.; Meier, D. Lithium-induced ECG modifications: Navigating from acute coronary syndrome to Brugada syndrome. BMJ Case Rep. CP 2021, 14, e241555. [Google Scholar] [CrossRef]
- Ott, M.; Stegmayr, B.; Salander Renberg, E.; Werneke, U. Lithium intoxication: Incidence, clinical course and renal function—A population-based retrospective cohort study. J. Psychopharmacol. 2016, 30, 1008–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245. [Google Scholar] [CrossRef] [Green Version]
- Neslund-Dudas, C.; Kandegedara, A.; Kryvenko, O.N.; Gupta, N.; Rogers, C.; Rybicki, B.A.; Dou, Q.P.; Mitra, B. Prostate tissue metal levels and prostate cancer recurrence in smokers. Biol. Trace Elem. Res. 2014, 157, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Coudon, T.; Hourani, H.; Nguyen, C.; Faure, E.; Mancini, F.R.; Fervers, B.; Salizzoni, P. Assessment of long-term exposure to airborne dioxin and cadmium concentrations in the Lyon metropolitan area (France). Environ. Int. 2018, 111, 177–190. [Google Scholar] [CrossRef]
- Wallace, D.R. Nanotoxicology and metalloestrogens: Possible involvement in breast cancer. Toxics 2015, 3, 390–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Authority, E.F.S. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597. [Google Scholar]
- Zimta, A.-A.; Schitcu, V.; Gurzau, E.; Stavaru, C.; Manda, G.; Szedlacsek, S.; Berindan-Neagoe, I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ. Res. 2019, 178, 108700. [Google Scholar] [CrossRef]
- Fischer, R.S.; Unrine, J.M.; Vangala, C.; Sanderson, W.T.; Mandayam, S.; Murray, K.O. Evidence of nickel and other trace elements and their relationship to clinical findings in acute Mesoamerican Nephropathy: A case-control analysis. PLoS ONE 2020, 15, e0240988. [Google Scholar] [CrossRef]
- Bruehlmeier, M.; Leenders, K.L.; Vontobel, P.; Calonder, C.; Antonini, A.; Weindl, A. Increased cerebral iron uptake in Wilson’s disease: A 52Fe-citrate PET study. J. Nucl. Med. 2000, 41, 781–787. [Google Scholar] [PubMed]
- Tomska, N.; Kosik-Bogacka, D.I.; Łanocha-Arendarczyk, N.; Szylińska, A.; Kotfis, K.; Sipak-Szmigiel, O.; Rotter, I. Relationship between concentrations of elements and geographic location in Poland. Ann. Agric. Environ. Med. 2021, 28, 283–290. [Google Scholar] [CrossRef]
- Brozoska, M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zink in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Exley, C. The toxicity of aluminium in humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Sheikh, S.; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef] [Green Version]
- Quintanar, L.; Lim, M.H. Metal Ions and Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Poulson, B.G.; Szczepski, K.; Lachowicz, J.I.; Jaremko, L.; Emwas, A.-H.; Jaremko, M. Aggregation of biologically important peptides and proteins: Inhibition or acceleration depending on protein and metal ion concentrations. RSC Adv. 2020, 10, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Emwas, A.-H.; Alghrably, M.; Dhahri, M.; Sharfalddin, A.; Alsiary, R.; Jaremko, M.; Faa, G.; Campagna, M.; Congiu, T.; Piras, M. Living with the enemy: From protein-misfolding pathologies we know, to those we want to know. Ageing Res. Rev. 2021, 70, 101391. [Google Scholar] [CrossRef] [PubMed]
- Piloni, N.E.; Fermandez, V.; Videla, L.A.; Puntarulo, S. Acute iron overload and oxidative stress in brain. Toxicology 2013, 314, 174–182. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Wandt, V.K.; Winkelbeiner, N.; Bornhorst, J.; Witt, B.; Raschke, S.; Simon, L.; Ebert, F.; Kipp, A.P.; Schwerdtle, T. A matter of concern–Trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021, 41, 101877. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, C.; Blicharska, E.; Krukow, P.; Jonak, K.; Maciejewski, M.; Szczepanek, D.; Jonak, K.; Flieger, J.; Maciejewski, R. Analysis of trace elements in human brain: Its aim, methods, and concentration levels. Front. Chem. 2019, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef] [PubMed]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-D.; Zhang, W.; Wang, Z.-Y.; Zhao, P. Serum copper, zinc, and iron levels in patients with Alzheimer’s disease: A meta-analysis of case-control studies. Front. Aging Neurosci. 2017, 9, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper dyshomeostasis in neurodegenerative diseases—Therapeutic implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper ions and Parkinson’s disease: Why is homeostasis so relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Dodani, S.C.; Domaille, D.W.; Nam, C.I.; Miller, E.W.; Finney, L.A.; Vogt, S.; Chang, C.J. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 5980–5985. [Google Scholar] [CrossRef] [Green Version]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Nurchi, V.M.; Crisponi, G.; Cappai, I.; Cappai, R.; Busato, M.; Melchior, A.; Tolazzi, M.; Peana, M.; Garribba, E. para-Aminosalicylic acid in the treatment of manganese toxicity. Complexation of Mn 2+ with 4-amino-2-hydroxybenzoic acid and its N-acetylated metabolite. New J. Chem. 2018, 42, 8035–8049. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Bornhorst, J.; Aschner, M.A. Manganese metabolism in humans. Front. Biosci. 2018, 711, 1655–1679. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Elem. Res. 2014, 161, 190–201. [Google Scholar] [CrossRef]
- Nakayama, A.; Hill, K.E.; Austin, L.M.; Motley, A.K.; Burk, R.F. All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J. Nutr. 2007, 137, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [Green Version]
- Angeli, J.P.F.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Marie, E.J.S. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 2019, 177, 1262–1279. [Google Scholar] [CrossRef] [Green Version]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.; Seubert, P. Links between long-term potentiation and neuropathology. An hypothesis involving calcium-activated proteases. Ann. N. Y. Acad. Sci. 1989, 568, 171–180. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Introduction and overview. Ann. N. Y. Acad. Sci. 1989, 568, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Toescu, E.C. Calcium and neuronal ageing. Trends Neurosci. 1998, 21, 2–7. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Europea, C. The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products of Nanotechnologies; European Commission: Strasbourg, France, 2006.
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, J.G.; Jain, P.K. Harvesting multiple electron–hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018, 10, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G. Challenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano 2018, 5, 48–63. [Google Scholar] [CrossRef] [Green Version]
- Pati, S.S.; Singh, L.H.; Guimarães, E.; Mantilla, J.; Coaquira, J.; Oliveira, A.; Sharma, V.K.; Garg, V.K. Magnetic chitosan-functionalized Fe3O4@ Au nanoparticles: Synthesis and characterization. J. Alloys Compd. 2016, 684, 68–74. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sayes, C.M.; Guo, B.; Pillai, S.; Parsons, J.G.; Wang, C.; Yan, B.; Ma, X. Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Sci. Total Environ. 2019, 653, 1042–1051. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, D.W.; Lee, Y.H.; Oh, J.H.; Yoon, S.; Choi, M.S.; Lee, S.K.; Kim, J.W.; Lee, K.; Song, C.-W. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch. Toxicol. 2011, 85, 1529–1540. [Google Scholar] [CrossRef]
- Pietruska, J.R.; Liu, X.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol. Sci. 2011, 124, 138–148. [Google Scholar] [CrossRef]
- Morawska, L.; Wang, H.; Ristovski, Z.; Jayaratne, E.; Johnson, G.; Cheung, H.; Ling, X.; He, C. JEM spotlight: Environmental monitoring of airborne nanoparticles. J. Environ. Monit. 2009, 11, 1758–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F., Jr. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557, 740–753. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Castellon, B.T.; Matson, C.W.; Aiken, G.R.; Hsu-Kim, H. Relative contributions of copper oxide nanoparticles and dissolved copper to Cu uptake kinetics of Gulf killifish (Fundulus grandis) embryos. Environ. Sci. Technol. 2017, 51, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Hu, X.; Zhou, Q.; Li, X.; Miao, X.; Zhou, R. Nanocolloids in natural water: Isolation, characterization, and toxicity. Environ. Sci. Technol. 2018, 52, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef]
- Wimmer, A.; Kalinnik, A.; Schuster, M. New insights into the formation of silver-based nanoparticles under natural and semi-natural conditions. Water Res. 2018, 141, 227–234. [Google Scholar] [CrossRef]
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural nanoparticles: Implications for environment and human health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Dong, S.; Qu, M.; Rui, Q.; Wang, D. Combinational effect of titanium dioxide nanoparticles and nanopolystyrene particles at environmentally relevant concentrations on nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 161, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanopart. Res. 2014, 16, 1–28. [Google Scholar] [CrossRef]
- Manfra, L.; Rotini, A.; Bergami, E.; Grassi, G.; Faleri, C.; Corsi, I. Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis. Ecotoxicol. Environ. Saf. 2017, 145, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [Green Version]
- Wilke, C.M.; Tong, T.; Gaillard, J.-F.; Gray, K.A. Attenuation of microbial stress due to nano-Ag and nano-TiO2 interactions under dark conditions. Environ. Sci. Technol. 2016, 50, 11302–11310. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.; Murashov, V.; Zumwalde, R.; Kuempel, E.; Geraci, C. Occupational exposure limits for nanomaterials: State of the art. J. Nanopart. Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. R. Soc. Interface 2010, 7 (Suppl. 1), S119–S129. [Google Scholar] [CrossRef] [Green Version]
- Elder, A.; Oberdörster, G. Translocation and effects of ultrafine particles outside of the lung. Clin. Occup. Environ. Med. 2006, 5, 785–796. [Google Scholar]
- Medina, C.; Santos-Martinez, M.; Radomski, A.; Corrigan, O.; Radomski, M. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef]
- Remelli, M.; Peana, M.; Medici, S.; Delogu, L.G.; Zoroddu, M.A. Interaction of divalent cations with peptide fragments from Parkinson’s disease genes. Dalton Trans. 2013, 42, 5964–5974. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Montanari, S.; Gatti, A.M. Nanopathology: The Health Impact of Nanoparticles; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gatti, A.M.; Bosco, P.; Rivasi, F.; Bianca, S.; Ettore, G.; Gaetti, L.; Montanari, S.; Bartoloni, G.; Gazzolo, D. Heavy metals nanoparticles in fetal kidney and liver tissues. Front. Biosci. 2011, 3, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, T.; Capone, S.; Gatti, A.; Capitani, F.; Cetta, F.; Palmieri, B. Intracellular heavy metal nanoparticle storage: Progressive accumulation within lymph nodes with transformation from chronic inflammation to malignancy. Int. J. Nanomed. 2010, 5, 955. [Google Scholar] [CrossRef] [Green Version]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Gatti, A.M.; Montanari, S. The side effects of drugs: Nanopathological hazards and risks. In Particles and Nanoparticles in Pharmaceutical Products; Springer: Berlin/Heidelberg, Germany, 2018; pp. 429–443. [Google Scholar]
- Snyder, W.; Cook, M.; Nasset, E.; Karhausen, L.; Howells, G.P.; Tipton, I. Report of the Task Group on Reference Man; ICRP Publication: Oxford, UK, 1975; Volume 23. [Google Scholar]
- Locci, E.; Pilia, I.; Piras, R.; Pili, S.; Marcias, G.; Cocco, P.; De Giorgio, F.; Bernabei, M.; Brusadin, V.; Allegrucci, L. Particle Background Levels in Human Tissues—PABALIHT project. Part I: A nanometallomic study of metal-based micro-and nanoparticles in liver and kidney in an Italian population group. J. Nanopart. Res. 2019, 21, 45. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.; Elisaf, M. Blood pressure drug therapy and electrolyte disturbances. Int. J. Clin. Pract. 2008, 62, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.A.; Rosenfeldt, F. Pharmaco-nutrient Interactions—A systematic review of zinc and antihypertensive therapy. Int. J. Clin. Pract. 2013, 67, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, J.I.; Nurchi, V.M.; Crisponi, G.; de Guadalupe Jaraquemada-Pelaez, M.; Caltagirone, C.; Peana, M.; Zoroddu, M.A.; Szewczuk, Z.; Cooper, G.J. Complex formation equilibria of Cu2+ and Zn2+ with Irbesartan and Losartan. Eur. J. Pharm. Sci. 2017, 97, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Karges, W.; Rink, L. Zinc and Diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85, 269S–276S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, C.G.; Martell, A.E. The Design of Chelating Agents for the Treatment of Iron Overload; ACS Publications: Washington, DC, USA, 1980. [Google Scholar] [CrossRef]
- Saran, B.M.; Russell, G.F. The effects of administering lithium carbonate on the balance of Na, K and water in manic-depressive patients. Psychol. Med. 1976, 6, 381–392. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Shrestha, K.P. Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. Biol. Trace Elem. Res. 1990, 25, 105–113. [Google Scholar] [CrossRef]
- Ohgami, H.; Terao, T.; Shiotsuki, I.; Ishii, N.; Iwata, N. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry 2009, 194, 464–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fels, A. Should we all take a bit of lithium. New York Times, 14 September 2014. [Google Scholar]
- Finley, P.R. Drug interactions with lithium: An update. Clin. Pharmacokinet. 2016, 55, 925–941. [Google Scholar] [CrossRef]
- Vasantha, P.; Shekhar, B.; PV, A.L. Copper-metformin ternary complexes: Thermal, photochemosensitivity and molecular docking studies. Mater. Sci. Eng. C 2018, 90, 621–633. [Google Scholar]
- Zhu, B.-Z.; Mao, L.; Fan, R.-M.; Zhu, J.-G.; Zhang, Y.-N.; Wang, J.; Kalyanaraman, B.; Frei, B. Ergothioneine prevents copper-induced oxidative damage to DNA and protein by forming a redox-inactive Ergothioneine—Copper complex. Chem. Res. Toxicol. 2011, 24, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, S.; Alghrably, M.; Campagna, M.; Hauser, C.A.E.; Jaremko, M.; Lachowicz, J.I. Metal Complex Formation and Anticancer Activity of Cu (I) and Cu (II) Complexes with Metformin. Molecules 2021, 26, 4730. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Hudecek, R.; Stejskal, J.; Sterzl, I. Diagnosis and treatment of metal-induced side-effects. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 7–16. [Google Scholar] [PubMed]
- Weiss, N.S.; LIFF, J.M. Accounting for the multicausal nature of disease in the design and analysis of epidemiologic studies. Am. J. Epidemiol. 1983, 117, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Sirlin, C.B.; Reeder, S.B. Magnetic resonance imaging quantification of liver iron. Magn. Reson. Imaging Clin. 2010, 18, 359–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biglia, A.; Morandi, V.; Monti, S.; Delvino, P.; Cavagna, L.; Montecucco, C. Cobalt hip prosthesis intoxication mimicking an autoimmune disease. Jt. Bone Spine 2020, 87, 652–654. [Google Scholar] [CrossRef]
- Pornwilard, M.; Weiskirchen, R.; Gassler, N.; Bosserhoff, A.K.; Becker, J.S. Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS ONE 2013, 8, e58702. [Google Scholar]
- Susnea, I.; Weiskirchen, R. Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom. Rev. 2016, 35, 666–686. [Google Scholar] [CrossRef]
- Henderson, R.; Hobbie, J.; Landrigan, P.; Mattisoti, D.; Perera, F.; Pfttaer, E.; Silbergeld, E.; Wogan, G. Biological markers in environmental health research. Environ. Health Perspect. 1987, 7, 3–9. [Google Scholar]
- Westphal, G.; Schnuch, A.; Schulz, T.; Reich, K.; Aberer, W.; Brasch, J.; Koch, P.; Wessbecher, R.; Szliska, C.; Bauer, A. Homozygous gene deletions of the glutathione S-transferases M1 and T1 are associated with thimerosal sensitization. Int. Arch. Occup. Environ. Health 2000, 73, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Gottberg, I.; Holmgren, B.; Jonsson, T.; Karlsson, G. Individual mercury exposure of chloralkali workers and its relation to blood and urinary mercury levels. Scand. J. Work Environ. Health 1979, 5, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, L.R.; Shannon, M.W.; Committee on Environmental Health. Technical report: Mercury in the environment: Implications for pediatricians. Pediatrics 2001, 108, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Hegde, A.; Shetty, G.; Jayasheelan, N. The Perturbation Encompassing Dental Amalgam Toxicity: A Review. Indian J. Forensic Med. Toxicol. 2020, 14, 147–152. [Google Scholar]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Hong, Y.-S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonenfant, J.; Miller, G.; Roy, P. Quebec beer-drinkers’ cardiomyopathy: Pathological studies. Can. Med. Assoc. J. 1967, 97, 910. [Google Scholar]
- Linna, A.; Oksa, P.; Groundstroem, K.; Halkosaari, M.; Palmroos, P.; Huikko, S.; Uitti, J. Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart. Occup. Environ. Med. 2004, 61, 877–885. [Google Scholar] [CrossRef]
- Peters, R.M.; Willemse, P.; Rijk, P.C.; Hoogendoorn, M.; Zijlstra, W.P. Fatal cobalt toxicity after a non-metal-on-metal total hip arthroplasty. Case Rep. Orthop. 2017, 2017, 9123684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paustenbach, D.J.; Galbraith, D.A.; Finley, B.L. Interpreting cobalt blood concentrations in hip implant patients. Clin. Toxicol. 2014, 52, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Ambardekar, A.V.; Devaraj, K.M.; Maleszewski, J.J.; Wolfel, E.E. Missing elements of the history. N. Engl. J. Med. 2014, 370, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.; Holland, J.; Quigley, L.; Sprague, S.; Bhandari, M. Pseudotumors associated with total hip arthroplasty. JBJS 2012, 94, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Skaug, M.A.; Cao, Y.; Andersen, O. Chelation in metal intoxication—principles and paradigms. J. Trace Elem. Med. Biol. 2015, 31, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Foreman, H.; Hamilton, J.G. The Use of Chelating Agents for Accelerating Excretion of Radioelements; US Atomic Energy Commission, Technical Information Service: San Francisco, CA, USA, 1951; Volume 3247.
- Soares, F.A.; Farina, M.; Santos, F.W.; Souza, D.; Rocha, J.B.T.; Nogueira, C.W. Interaction between metals and chelating agents affects glutamate binding on brain synaptic membranes. Neurochem. Res. 2003, 28, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 1999, 99, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Modell, W.; Gold, H.; Cattell, M. Clinical uses of 2,3-dimercaptopropanol (BAL). IV. Pharmacologic observations on BAL by intramuscular injection in man. J. Clin. Investig. 1946, 25, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilensky, J.A.; Redman, K. British anti-Lewisite (dimercaprol): An amazing history. Ann. Emerg. Med. 2003, 41, 378–383. [Google Scholar] [CrossRef]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.; Ball, H.J.; Hare, D.J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeary, F.A.; Rcom-H’cheo-Gauthier, A.N.; Goulding, M.; Radford, R.A.; Okita, Y.; Faller, P.; Chung, R.S.; Pountney, D.L. Switching on Endogenous metal binding proteins in Parkinson’s Disease. Cells 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.M.; Bohic, S.; Carmona, A.; Ortega, R.; Cottam, V.; Hare, D.J.; Finberg, J.P.; Reyes, S.; Halliday, G.M.; Mercer, J.F. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging 2014, 35, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Tórsdóttir, G.; Kristinsson, J.; Sveinbjörnsdóttir, S.; Snaedal, J.; Jóhannesson, T. Copper, ceruloplasmin, superoxide dismutase and iron parameters in Parkinson’s disease. Pharmacol. Toxicol. 1999, 85, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s disease: Mechanisms and biochemical processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Karri, V.; Tay, N.W.R.; Chang, K.H.; Ah, H.Y.; Ng, P.Q.; San Ho, H.; Keh, H.W.; Candasamy, M. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed. Pharmacother. 2019, 111, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Di Marco, V. Metal chelation therapy and Parkinson’s disease: A critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, G.; Santoro, A.M.; Lanza, V.; Sbardella, D.; Tundo, G.R.; Ciaccio, C.; Marini, S.; Coletta, M.; Milardi, D. The double faced role of copper in Aβ homeostasis: A survey on the interrelationship between metal dyshomeostasis, UPS functioning and autophagy in neurodegeneration. Coord. Chem. Rev. 2017, 347, 1–22. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Watanabe, R.; Haratake, M.; Nakayama, M.; Saji, H. Diphenylpropynone derivatives as probes for imaging β-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. Lett. 2011, 21, 117–120. [Google Scholar] [CrossRef]
- Jones, M.R.; Mathieu, E.; Dyrager, C.; Faissner, S.; Vaillancourt, Z.; Korshavn, K.J.; Lim, M.H.; Ramamoorthy, A.; Yong, V.W.; Tsutsui, S. Multi-target-directed phenol–triazole ligands as therapeutic agents for Alzheimer’s disease. Chem. Sci. 2017, 8, 5636–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gomes, L.M.; Zhang, T.; Storr, T. A small bifunctional chelator that modulates Aβ42 aggregation. Can. J. Chem. 2018, 96, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kochi, A.; Pithadia, A.S.; Lee, S.; Nam, Y.; Beck, M.W.; He, X.; Lee, D.; Lim, M.H. Tuning reactivity of diphenylpropynone derivatives with metal-associated amyloid-β species via structural modifications. Inorg. Chem. 2013, 52, 8121–8130. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, J.; Xu, R.; Song, Q.; Zhang, X.; Liu, H.; Qiang, X.; Li, Y.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of 4′-OH-flurbiprofen-chalcone hybrids as potential multifunctional agents for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2018, 26, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Fosso, M.Y.; LeVine, H., 3rd; Green, K.D.; Tsodikov, O.V.; Garneau-Tsodikova, S. Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones. Org. Biomol. Chem. 2015, 13, 9418–9426. [Google Scholar] [CrossRef]
- Schugar, H.; Green, D.E.; Bowen, M.L.; Scott, L.E.; Storr, T.; Böhmerle, K.; Thomas, F.; Allen, D.D.; Lockman, P.R.; Merkel, M. Combating Alzheimer’s disease with multifunctional molecules designed for metal passivation. Angew. Chem. 2007, 119, 1746–1748. [Google Scholar] [CrossRef]
- Telpoukhovskaia, M.A.; Cawthray, J.F.; Rodríguez-Rodríguez, C.; Scott, L.E.; Page, B.D.; Patrick, B.O.; Orvig, C. 3-Hydroxy-4-pyridinone derivatives designed for fluorescence studies to determine interaction with amyloid protein as well as cell permeability. Bioorg. Med. Chem. Lett. 2015, 25, 3654–3657. [Google Scholar] [CrossRef]
- Green, D.E.; Bowen, M.L.; Scott, L.E.; Storr, T.; Merkel, M.; Böhmerle, K.; Thompson, K.H.; Patrick, B.O.; Schugar, H.J.; Orvig, C. In vitro studies of 3-hydroxy-4-pyridinones and their glycosylated derivatives as potential agents for Alzheimer’s disease. Dalton Trans. 2010, 39, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, P.; Liu, Q.; Wu, J.; Yin, Y.; Wang, X.; Kong, L. Novel 8-hydroxyquinoline derivatives targeting β-amyloid aggregation, metal chelation and oxidative stress against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.; Vieira, R.P.; Jones, M.R.; Wang, M.C.; Dyrager, C.; Souza-Fagundes, E.M.; Da Silva, J.G.; Storr, T.; Beraldo, H. 8-Hydroxyquinoline Schiff-base compounds as antioxidants and modulators of copper-mediated Aβ peptide aggregation. J. Inorg. Biochem. 2014, 139, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hu, J.; Yang, X.; Feng, X.; Li, X.; Huang, L.; Chan, A.S. Design, synthesis, and evaluation of orally bioavailable quinoline–indole derivatives as innovative multitarget-directed ligands: Promotion of cell proliferation in the adult murine Hippocampus for the treatment of alzheimer’s disease. J. Med. Chem. 2018, 61, 1871–1894. [Google Scholar] [CrossRef]
- Zheng, H.; Youdim, M.B.; Fridkin, M. Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective− neurorestorative moieties for Alzheimer’s therapy. J. Med. Chem. 2009, 52, 4095–4098. [Google Scholar] [CrossRef]
- Oliveri, V.; Grasso, G.I.; Bellia, F.; Attanasio, F.; Viale, M.; Vecchio, G. Soluble sugar-based quinoline derivatives as new antioxidant modulators of metal-induced amyloid aggregation. Inorg. Chem. 2015, 54, 2591–2602. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Zhu, S.; Gu, X.; Jia, X.; Lu, Y.; Zhu, L. Two macrocyclic polyamines as modulators of metal-mediated Aβ40 aggregation. Integr. Biol. 2015, 7, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Lanza, V.; D’Agata, R.; Iacono, G.; Bellia, F.; Spoto, G.; Vecchio, G. Cyclam glycoconjugates as lectin ligands and protective agents of metal-induced amyloid aggregation. J. Inorg. Biochem. 2015, 153, 377–382. [Google Scholar] [CrossRef]
- Lincoln, K.M.; Gonzalez, P.; Richardson, T.E.; Julovich, D.A.; Saunders, R.; Simpkins, J.W.; Green, K.N. A potent antioxidant small molecule aimed at targeting metal-based oxidative stress in neurodegenerative disorders. Chem. Commun. 2013, 49, 2712–2714. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, K.M.; Richardson, T.E.; Rutter, L.; Gonzalez, P.; Simpkins, J.W.; Green, K.N. An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation. ACS Chem. Neurosci. 2012, 3, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, P.; Da Costa, V.C.; Hyde, K.; Wu, Q.; Annunziata, O.; Rizo, J.; Akkaraju, G.; Green, K.N. Bimodal-hybrid heterocyclic amine targeting oxidative pathways and copper mis-regulation in Alzheimer’s disease. Metallomics 2014, 6, 2072–2082. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Finkelstein, D.; Hawco, N.J.; Rath, N.P.; Kim, J.; Mirica, L.M. Bifunctional compounds for controlling metal-mediated aggregation of the Aβ42 peptide. J. Am. Chem. Soc. 2012, 134, 6625–6636. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.R.; Mu, C.; Wang, M.C.; Webb, M.I.; Walsby, C.J.; Storr, T. Modulation of the Aβ peptide aggregation pathway by KP1019 limits Aβ-associated neurotoxicity. Metallomics 2015, 7, 129–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.-Y.; Yin, W.-X.; Yin, J.; Zhang, S.-B.; Liu, C.-L. The chelation targeting metal–Aβ40 aggregates may lead to formation of Aβ40 oligomers. Dalton Trans. 2011, 40, 4830–4833. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Telpoukhovskaia, M.; Alí-Torres, J.; Rodríguez-Santiago, L.; Manso, Y.; Bailey, G.; Hidalgo, J.; Sodupe, M.; Orvig, C. Thioflavin-based molecular probes for application in Alzheimer’s disease: From in silico to in vitro models. Metallomics 2015, 7, 83–92. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Sanchez de Groot, N.; Rimola, A.; Alvarez-Larena, A.; Lloveras, V.; Vidal-Gancedo, J.; Ventura, S.; Vendrell, J.; Sodupe, M.; González-Duarte, P. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J. Am. Chem. Soc. 2009, 131, 1436–1451. [Google Scholar] [CrossRef]
- Viveiros, R.; Karim, K.; Piletsky, S.; Heggie, W.; Casimiro, T. Development of a molecularly imprinted polymer for a pharmaceutical impurity in supercritical CO2: Rational design using computational approach. J. Clean. Prod. 2017, 168, 1025–1031. [Google Scholar] [CrossRef]
- Beck, M.W.; Derrick, J.S.; Kerr, R.A.; Oh, S.B.; Cho, W.J.; Lee, S.J.C.; Ji, Y.; Han, J.; Tehrani, Z.A.; Suh, N. Structure-mechanism-based engineering of chemical regulators targeting distinct pathological factors in Alzheimer’s disease. Nat. Commun. 2016, 7, 13115. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, M.; Sheng, R.; Ma, Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 174–186. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, X.-B.; Kong, L.-Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef]
- DeToma, A.S.; Krishnamoorthy, J.; Nam, Y.; Lee, H.J.; Brender, J.R.; Kochi, A.; Lee, D.; Onnis, V.; Congiu, C.; Manfredini, S. Interaction and reactivity of synthetic aminoisoflavones with metal-free and metal-associated amyloid-β. Chem. Sci. 2014, 5, 4851–4862. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Park, H.M.; Hyung, S.-J.; DeToma, A.S.; Kim, C.; Ruotolo, B.T.; Lim, M.H. Exploring the reactivity of flavonoid compounds with metal-associated amyloid-β species. Dalton Trans. 2012, 41, 6558–6566. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-M.; Cai, P.; Liu, Q.-H.; Xu, D.-Q.; Yang, X.-L.; Wu, J.-J.; Kong, L.-Y.; Wang, X.-B. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016, 123, 282–297. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.-M.; Wu, J.-J.; Wang, J.; Xie, S.-S.; Lan, J.-S.; Xu, W.; Kong, L.-Y.; Wang, X.-B. Synthesis and pharmacological evaluation of donepezil-based agents as new cholinesterase/monoamine oxidase inhibitors for the potential application against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. 3), 41–53. [Google Scholar] [CrossRef]
- Benchekroun, M.; Romero, A.; Egea, J.; Leon, R.; Michalska, P.; Buendía, I.; Jimeno, M.L.; Jun, D.; Janockova, J.; Sepsova, V. The antioxidant additive approach for Alzheimer’s disease therapy: New ferulic (lipoic) acid plus melatonin modified tacrines as cholinesterases inhibitors, direct antioxidants, and nuclear factor (erythroid-derived 2)-like 2 activators. J. Med. Chem. 2016, 59, 9967–9973. [Google Scholar] [CrossRef]
- Skibiński, R.; Czarnecka, K.; Girek, M.; Bilichowski, I.; Chufarova, N.; Mikiciuk-Olasik, E.; Szymański, P. Novel tetrahydroacridine derivatives with iodobenzoic acid moiety as multifunctional acetylcholinesterase inhibitors. Chem. Biol. Drug Des. 2018, 91, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, B.; Shi, Z.; Wang, X.; Lei, S.; Tang, X.; Liang, H.; Liu, Q.; Gong, M.; Peng, R. New tris (dopamine) derivative as an iron chelator. Synthesis, solution thermodynamic stability, and antioxidant research. J. Inorg. Biochem. 2017, 171, 29–36. [Google Scholar] [CrossRef]
- Bolognin, S.; Drago, D.; Messori, L.; Zatta, P. Chelation therapy for neurodegenerative diseases. Med. Res. Rev. 2009, 29, 547–570. [Google Scholar] [CrossRef]
- Chianella, C.; Gragnaniello, D.; Delser, P.M.; Visentini, M.F.; Sette, E.; Tola, M.R.; Barbujani, G.; Fuselli, S. BCHE and CYP2D6 genetic variation in Alzheimer’s disease patients treated with cholinesterase inhibitors. Eur. J. Clin. Pharmacol. 2011, 67, 1147–1157. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Karliński, M.; Dziezyc, K.; Chabik, G.; Czerska, M. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur. J. Neurol. 2014, 21, 599–606. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. Effect of an extract from Aronia melanocarpa L. berries on the body status of zinc and copper under chronic exposure to cadmium: An in vivo experimental study. Nutrients 2017, 9, 1374. [Google Scholar] [CrossRef] [Green Version]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.W.; Bush, A.I.; Masters, C.L. Metal-protein attenuating compounds and Alzheimer’s disease. Expert Opin. Investig. Drugs 2004, 13, 1585–1592. [Google Scholar] [CrossRef]
- Matlack, K.E.; Tardiff, D.F.; Narayan, P.; Hamamichi, S.; Caldwell, K.A.; Caldwell, G.A.; Lindquist, S. Clioquinol promotes the degradation of metal-dependent amyloid-β (Aβ) oligomers to restore endocytosis and ameliorate Aβ toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, 4013–4018. [Google Scholar] [CrossRef] [Green Version]

| Metal | Disease | Gene | Affected Tissue | Therapy |
|---|---|---|---|---|
| Mn | Manganese transporter deficiency | SLC30A10 SLC39A14 (manganese transporter) | Liver, Nervous system | Manganese(II)-sulfate monohydrate [10] |
| Fe | Aceruloplasminemia | CPL (Ceruloplasmin) CP (Ferroxidase) | Liver, pancreas, nervous system | Iron chelation (Deferoxamine, Deferasirox) + Vitamin E and C/+ Fresh Frozen Plasma, Zinc administration, Minocycline administration, Enzyme Replacement Therapy, Gene Therapy [11] |
| Neuroferritinopathy, Hyperferritinemia-cataract syndrome, L-ferritin deficiency | FTL (iron storage) | Nervous system | Iron chelation (Deferoxamine, Deferasirox), dopamine-related drugs [12] | |
| Spastic paraplegia type 35 | FA2H (fatty acid 2-hydroxylase (Synthesis of sphingolipids)) | Botulinum toxin injections, microtubule destabilizing drugs (e.g., vinblastine) [13] | ||
| HARP syndrome (hypoprebetalipoproteinemia, acanthocytosis, retinitis pigmentosa, and pallidal degeneration) | PANK2 (Pantothenate kinase (CoA synthesis)) | No therapy | ||
| Pontocerebellar hypoplasia type 12 | COASY (CoA synthesis) | |||
| Infantile neuroaxonal dystrophy 1, Neurodegeneration with brain iron accumulation 2B, Parkinson’s disease type 14 | PLA2G6 (Phospholipase) | |||
| Spastic paraplegia 43, Neurodegeneration with brain iron accumulation 4 | C19orf12 (Mitochondrial magnesium homeostasis) | Intrathecal baclofen [14] | ||
| Woodhouse–Sakati syndrome | DCAF17 (Ubiquitinylation) | Treatment is symptomatic (e.g., hormone replacement therapy for hypogonadism) [15] | ||
| Neurodegeneration with brain iron accumulation type 5 | WDR45 (Autophagy) | Treatment is symptomatic [16] | ||
| Kufor–Rakeb syndrome, Spastic paraplegia type 78 | ATP13A2 (Lysosomal divalent cation (transition metal) transporter) | Treatment is symptomatic [16] | ||
| Hereditary hemochromatosis | HFE1 (HFE protein), HJV (Hemojuvelin), TrR2 (Trasferrin receptor-2), SLC40A1 (Ferroportin), HAMP (Hepcidin) | Liver, pancreas, heart | Therapeutic phlebotomy, iron chelating therapy, erythropoietin [17] | |
| Cu | Wilson’s disease | ATP7B (beta polypeptide, ATPase, CuII transporting) | Liver, brain, kidneys, cornea | Copper chelation (e.g., Penicillamine, Trientine), zinc supplementation, Tetrathiomolybdate [18] |
| MEDNIK syndrome | AP1S1 (adaptor protein complex 1 subunit β1) | liver, nervous system | Zinc supplementation (e.g., zinc acetate) [19] | |
| Menkes Disease | ATP7A (ATPase Copper Transporting Alpha) | Nervous system, skeletal, skin | Copper supplementation (e.g., copper histidine) [20] | |
| Occipital Horn Syndrome (OHS) | ATP7A (P-type ATPase) | Nervous system, skeletal, skin | Copper supplementation, disulfiram [21] | |
| Huppke-Brendel Syndrome (HBS) | SLC33A1 | Nervous system | Treatment is symptomatic [22] | |
| Zn | Acrodermatitis Enteropathica | SLC39A4 (Solute Carrier Family 39 Member 4) | Liver | Zinc supplementation [23] |
| Transient Neonatal Zinc Deficiency | SLC30A2 (Solute carrier family 30 member 2) | Skin | Zinc replacement therapy [24] | |
| Ehlers-Danlos Syndrome | SLC39A13 (zinc transporter ZIP13) | Nervous system, Muscle, skeletal | Nutritional supplements [25] | |
| Birk-Landau-Perez Syndrome | SLC30A9 (Zinc transporter 9) | Nervous system, kidneys, | Symptomatic Therapy [26] | |
| Se | Keshan Disease | Under investigation, genes related to selenoproteins and thioredoxin reductase | Heart | Selenium supplementation [27] |
| Rigid spine muscular dystrophy 1 (RSMD1) and congenital myopathy with fiber-type disproportion | SEPN1 (Selenoprotein N) | Muscle nervous system | No approved drug therapies [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639. https://doi.org/10.3390/molecules26216639
Lachowicz JI, Lecca LI, Meloni F, Campagna M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules. 2021; 26(21):6639. https://doi.org/10.3390/molecules26216639
Chicago/Turabian StyleLachowicz, Joanna Izabela, Luigi Isaia Lecca, Federico Meloni, and Marcello Campagna. 2021. "Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy" Molecules 26, no. 21: 6639. https://doi.org/10.3390/molecules26216639
APA StyleLachowicz, J. I., Lecca, L. I., Meloni, F., & Campagna, M. (2021). Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules, 26(21), 6639. https://doi.org/10.3390/molecules26216639