Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea)
Abstract
:1. Introduction
2. Results and Discussion
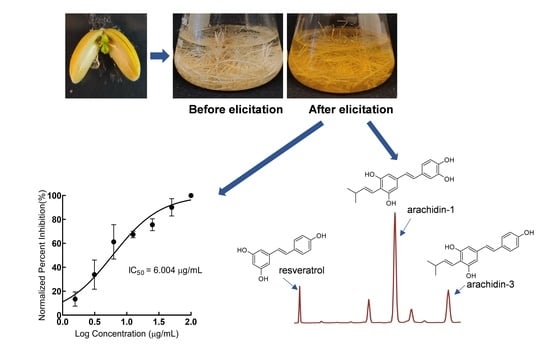
2.1. Development and Characterization of Peanut cv. Tifrunner Hairy Roots
2.2. Production of Prenylated Stilbenoids in Hairy Roots of Peanut cvs. Tifrunner, Hull, and Georgia Green
2.3. Comparison of Antioxidant Activity of Stilbenoid-Rich Extract from Hairy Roots of Three Peanut Cultivars
3. Materials and Methods
3.1. Seed Sterilization and Germination of Peanut cv. Tifrunner
3.2. Establishment of Hairy Root Cultures of Peanut cv. Tifrunner
3.3. Growth Conditions and Elicitation of Peanut Hairy Root Cultures of cvs. Tifrunner, Hull, and Georgia Green
3.4. Extraction and Analysis of Stilbenoids
3.5. DPPH Antioxidant Assay
3.6. Purification and Identification of Arachidin-6 in Peanut Hairy Root Culture
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.Y.; Whiteman, M.; Peng, Z.F.; Jenner, A.; Yong, E.L.; Halliwell, B. Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional chinese medicines. Free Radic. Biol. Med. 2004, 36, 1575–1587. [Google Scholar] [CrossRef]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef]
- Dávid, C.Z.; Hohmann, J.; Vasas, A. Chemistry and pharmacology of Cyperaceae stilbenoids: A Review. Molecules 2021, 26, 2794. [Google Scholar] [CrossRef]
- Sobolev, V.S. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008, 56, 1949–1954. [Google Scholar] [CrossRef]
- Sobolev, V.S. Production of phytoalexins in peanut (Arachis hypogaea) seed elicited by selected microorganisms. J. Agric. Food Chem. 2013, 61, 1850–1858. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, V.S.; Potter, T.L.; Horn, B.W. Prenylated stilbenes from peanut root mucilage. Phytochem. Anal. 2006, 17, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L.; Huang, D. Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J. Agric. Food Chem. 2011, 59, 5993–6003. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Thallapuranam, S.K.; Mockaitis, K.; Medina-Bolivar, F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Fang, L.; Medina-Bolivar, F. Production and biosynthesis of bioactive stilbenoids in hairy root cultures. In Production of Plant Derived Natural Compounds through Hairy Root Culture; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 45–64. [Google Scholar]
- Brents, L.; Medina-Bolivar, F.; Seely, K.; Nair, V.; Bratton, S.; Ñopo-Olazabal, L.; Patel, R.; Liu, H.; Doerksen, R.; Prather, P.; et al. Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 2011, 42, 139–156. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-C.; Lai, Y.-H.; Djoko, B.; Wu, P.-L.; Liu, C.-D.; Liu, Y.-W.; Chiou, R.Y.-Y. Biosynthesis enhancement and antioxidant and anti-inflammatory activities of peanut (Arachis hypogaea L.) arachidin-1, arachidin-3, and isopentadienylresveratrol. J. Agric. Food Chem. 2006, 54, 10281–10287. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-P.; Au, L.-C.; Chiou, R.Y.-Y.; Chung, P.-C.; Chen, S.-Y.; Tang, W.-C.; Chang, C.-L.; Fang, W.-H.; Lin, S.-B. Arachidin-1, a peanut stilbenoid, induces programmed cell death in human leukemia HL-60 cells. J. Agric. Food Chem. 2010, 58, 12123–12129. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid Compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef]
- Fang, L.; Yang, T.; Medina-Bolivar, F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef] [Green Version]
- Chayjarung, P.; Poonsap, W.; Pankaew, C.; Inmano, O.; Kongbangkerd, A.; Limmongkon, A. Using a combination of chitosan, methyl jasmonate, and cyclodextrin as an effective elicitation strategy for prenylated stilbene compound production in Arachis hypogaea L. hairy root culture and their impact on genomic DNA. Plant Cell Tissue Organ Cult. 2021, 147, 117–129. [Google Scholar] [CrossRef]
- Agarwal, G.; Clevenger, J.; Pandey, M.K.; Wang, H.; Shasidhar, Y.; Chu, Y.; Fountain, J.C.; Choudhary, D.; Culbreath, A.K.; Liu, X.; et al. High-density genetic map using whole-genome resequencing for fine mapping and candidate gene discovery for disease resistance in peanut. Plant Biotechnol. J. 2018, 16, 1954–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bruijn, W.J.C.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.-P. Antibacterial prenylated stilbenoids from peanut (Arachis hypogaea). Phytochem. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Limmongkon, A.; Nopprang, P.; Chaikeandee, P.; Somboon, T.; Wongshaya, P.; Pilaisangsuree, V. LC-MS/MS profiles and interrelationships between the anti-inflammatory activity, total phenolic content and antioxidant potential of Kalasin 2 cultivar peanut sprout crude extract. Food Chem. 2018, 239, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Pilaisangsuree, V.; Anuwan, P.; Supdensong, K.; Lumpa, P.; Kongbangkerd, A.; Limmongkon, A. Enhancement of adaptive response in peanut hairy root by exogenous signalling molecules under cadmium stress. J. Plant Physiol. 2020, 254, 153278. [Google Scholar] [CrossRef] [PubMed]
- Wongshaya, P.; Chayjarung, P.; Tothong, C.; Pilaisangsuree, V.; Somboon, T.; Kongbangkerd, A.; Limmongkon, A. Effect of light and mechanical stress in combination with chemical elicitors on the production of stilbene compounds and defensive responses in peanut hairy root culture. Plant Physiol. Biochem. 2020, 157, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N.D. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Cheng, P.; Chiu, P.; Chang, J.; Lin, S.; Li, Y.; Lo, D.; Lai, L.; Wu, S.; Chiou, R. Inhibition of testosterone-mediated benign prostatic enlargement of orchiectomized Sprague-Dawley rats by diets supplemented with bio-elicited peanut sprout powder (BPSP) and three new BPSP-extracted natural compounds identified. J. Funct. Foods 2021, 79, 104383. [Google Scholar] [CrossRef]
- Kim, S.; Seo, J.; Kim, B.; Kim, H.; Lee, H.; Kim, J. Anti-obesity activity of peanut sprout extract. Food Sci. Biotechnol. 2014, 23, 601–607. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef]
- Balmaceda, C. Efecto de la Cicodextrina y el Metil Jamonato en la Producción de Resveratrol y sus Análogos Prenilados Araquidina-1 y Araquidina-3 Empleando Raices en Cabellera de Mani. Licentiate Thesis, Universidad Peruana Cayetano Heredia, Lima, Peru, 2011. [Google Scholar]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef]
- Shrivastava, A. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Ball, J.M.; Medina-Bolivar, F.; Defrates, K.; Hambleton, E.; Hurlburt, M.E.; Fang, L.; Yang, T.; Nopo-Olazabal, L.; Atwill, R.L.; Ghai, P.; et al. Investigation of stilbenoids as potential therapeutic agents for rotavirus gastroenteritis. Adv. Virol. 2015, 2015, 293524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Roberto, P. Antioxidant Characterization of Peanut Hairy Roots Extracts Enriched with Prenylated Stilbenoids. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 2020. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]







| tR (Min) | UV Max (nm) | [M − H]− | MS2 Ions a | MS3 Ions | [M + H]+ | MS2 Ions a | MS3 Ions |
|---|---|---|---|---|---|---|---|
| 16.57 | 344 | 309 | 291, 265, 294 | 159, 249 | 311 | 201, 283, 296 | 159, 173, 183 |
| Stilbenoids | µg/mg DW a | ||
|---|---|---|---|
| Tifrunner | Hull | Georgia Green | |
| Resveratrol | 29.47 ± 1.40 | 60.56 ± 1.19 | 12.52 ± 0.29 |
| Arachidin-5 | 30.92 ± 1.52 | 13.5 ± 0.29 | 6.9 ± 0.13 |
| Arachidin-1 | 207.5 ± 7.35 | 162.37 ± 1.33 | 108.76 ± 1.53 |
| Arachidin-2 | 39.15 ± 0.98 | 28.21 ± 1.97 | 19.74 ± 2.21 |
| Arachidin-3 | 75.28 ± 7.39 | 72.24 ± 2.05 | 46.78 ± 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajurel, G.; Hasan, R.; Medina-Bolivar, F. Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea). Molecules 2021, 26, 6778. https://doi.org/10.3390/molecules26226778
Gajurel G, Hasan R, Medina-Bolivar F. Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea). Molecules. 2021; 26(22):6778. https://doi.org/10.3390/molecules26226778
Chicago/Turabian StyleGajurel, Gaurav, Rokib Hasan, and Fabricio Medina-Bolivar. 2021. "Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea)" Molecules 26, no. 22: 6778. https://doi.org/10.3390/molecules26226778
APA StyleGajurel, G., Hasan, R., & Medina-Bolivar, F. (2021). Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea). Molecules, 26(22), 6778. https://doi.org/10.3390/molecules26226778