Synthesis, Structure, and Photophysical Properties of Yellow-Green and Blue Photoluminescent Dinuclear and Octanuclear Copper(I) Iodide Complexes with a Disilanylene-Bridged Bispyridine Ligand
Abstract
:1. Introduction
2. Results and Discussion
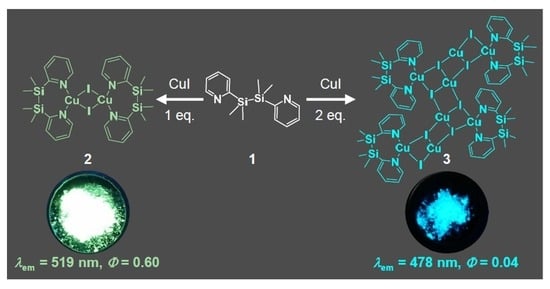
2.1. Synthesis and Crystal Structure of Cu Complexes 2 and 3
2.2. Photophysical Properties
2.3. Theoretical Consideration
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wallesch, M.; Volz, D.; Zink, D.M.; Schepers, U.; Nieger, M.; Baumann, T.; Bräse, S. Bright Coppertunities: Multinuclear CuI Complexes with N–P Ligands and Their Applications. Chem. Eur. J. 2014, 20, 6578–6590. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(i)–phenanthrolines. A viable alternative to Ru(ii)–polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Tsubomura, T.; Tsukuda, T.; Matsumoto, K. Luminescent d10 transition metal complexes. Bull. Jpn. Soc. Coord. Chem. 2008, 52, 29–42. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes–Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper(I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- McMillin, D.R.; McNett, K.M. Photoprocesses of Copper Complexes That Bind to DNA. Chem. Rev. 1998, 98, 1201–1220. [Google Scholar] [CrossRef] [PubMed]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A. TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes. Chem. Phys. Chem. 2017, 18, 3508–3535. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and Photophysics of Coordination Compounds: Copper. In Photochemistry and Photophysics of Coordination Compounds I.; Balzani, V., Campagna, S., Eds.; Springer: Berlin, Germany, 2007; Volume 280, pp. 69–115. [Google Scholar]
- Peng, R.; Li, M.; Li, D. Copper(I) halides: A versatile family in coordination chemistry and crystal engineering. Coord. Chem. Rev. 2010, 254, 1–18. [Google Scholar] [CrossRef]
- Blake, A.J.; Brooks, N.R.; Champness, N.R.; Hanton, L.R.; Hubberstey, P.; Schroder, M. Copper(I) Halide Supramolecular Networks Linked by N-heterocyclic Donor Bridging Ligands. Pure Appl. Chem. 1998, 70, 2351–2357. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Huang, W.; Liu, X. Recent progress in Metal-Organic Complexes for Optoelectronic Applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yiu, S.-C.; Ho, C.-L.; Wong, W.-Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Vitale, M.; Ford, P.C. Luminescent mixed ligand copper(I) clusters (CuI)n(L)m (L = pyridine, piperidine): Thermodynamic control of molecular and supramolecular species. Coord. Chem. Rev. 2001, 219–221, 3–16. [Google Scholar] [CrossRef]
- Araki, H.; Tsuge, K.; Sasaki, Y.; Ishizaka, S.; Kitamura, N. Luminescence Ranging from Red to Blue: A Series of Copper(I)-Halide Complexes Having Rhombic {Cu2(μ-X)2} (X = Br and I) Units with N-Heteroaromatic Ligands. Inorg. Chem. 2005, 44, 9667–9675. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Wang, S.; Liu, W. Copper iodide organic-inorganic hybrid chelating clusters as luminescent coating materials. Inorg. Chim. Acta 2021, 518, 120241. [Google Scholar] [CrossRef]
- Starosta, R.; Puchalska, M.; Cybiṅska, J.; Barysa, M.; Mudring, A.V. Structures, electronic properties and solid state lumi-nescence of Cu(I) iodide complexes with 2,9-dimethyl-1,10-phenanthroline and aliphatic aminomethylphosphines or tri-phenylphosphine. Dalton Trans. 2011, 40, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Phifer, C.C.; McMillin, D.R. The basis of aryl substituent effects on charge-transfer absorption intensities. Inorg. Chem. 1986, 25, 1329–1333. [Google Scholar] [CrossRef]
- Miller, M.T.; Gantzel, P.K.; Karpishin, T.B. A Highly Emissive Heteroleptic Copper(I) Bis(phenanthroline) Complex: [Cu(dbp)(dmp)]+ (dbp = 2,9-Di-tert-butyl-1,10-phenanthroline; dmp = 2,9-Dimethyl-1,10-phenanthroline). J. Am. Chem. Soc. 1999, 121, 4292–4293. [Google Scholar] [CrossRef]
- Alkan-Zambada, M.; Constable, E.C.; Housecroft, C.E. The role of percent volume buried in the characterization of cop-per(I) complexes for lighting purposes. Molecules 2020, 25, 2647. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, M.K.; McMillin, D.R.; Koenig, K.S.; Pallenberg, A.J. Steric Effects in the Ground and Excited States of Cu(NN)2+ Systems. Inorg. Chem. 1997, 36, 172–176. [Google Scholar] [CrossRef]
- Omoto, K.; Nakae, T.; Nishio, M.; Yamanoi, Y.; Kasai, H.; Nishibori, E.; Mashimo, T.; Seki, T.; Ito, H.; Nakamura, K.; et al. Thermosalience in macrocycle-based soft crystals via anisotropic deformation of disilanyl ar-chitecture. J. Am. Chem. Soc. 2020, 142, 12651–12657. [Google Scholar] [CrossRef]
- Shimada, M.; Tsuchiya, M.; Sakamoto, R.; Yamanoi, Y.; Nishibori, E.; Sugimoto, K.; Nishihara, H. Bright Solid-State Emis-sion of Disilane-Bridged Donor‒Acceptor‒Donor and Acceptor‒Donor‒Acceptor Chromophores. Angew. Chem. Int. Ed. 2016, 55, 3022–3026. [Google Scholar] [CrossRef] [PubMed]
- Nakae, T.; Nishio, M.; Usuki, T.; Ikeya, M.; Nishimoto, C.; Ito, S.; Maeda, H.; Nishihara, H.; Hattori, M.; Jimura, K.; et al. Luminescent behavior elucidation of disilane-bridged D‒A‒D triad composed of phenothiazine and thienopyradine. Angew. Chem. Int. Ed. 2021, 60, 22871–22878. [Google Scholar] [CrossRef]
- Hirata, S.; Nishio, M.; Uchida, H.; Usuki, T.; Nakae, T.; Miyachi, M.; Yamanoi, Y.; Nishihara, H. Effect of the Tris(trimethylsilyl)silyl Group on the Fluorescence and Triplet Yields of Oligothiophenes. J. Phys. Chem. C 2020, 124, 3277–3286. [Google Scholar] [CrossRef]
- Usuki, T.; Omoto, K.; Shimada, M.; Yamanoi, Y.; Kasai, H.; Nishibori, E.; Nishihara, H. Effects of Substituents on the Blue Luminescence of Disilane-Linked Donor‒Acceptor‒Donor Triads. Molecules 2019, 24, 521. [Google Scholar] [CrossRef] [Green Version]
- Usuki, T.; Shimada, M.; Yamanoi, Y.; Ohto, T.; Tada, H.; Kasai, H.; Nishibori, E.; Nishihara, H. Aggregation-induced En-hanced Emission from Disilane bridged Donor‒Acceptor‒Donor Luminogens Based on Triarylamine Functionality. ACS Appl. Mater. Interfaces 2018, 10, 12164–12172. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Ohto, T.; Pham, S.-T.; Yamada, R.; Tada, H.; Omoto, K.; Tashiro, S.; Shionoya, M.; Hattori, M.; et al. Multifunctional Octamethyltetrasila[2.2]cyclophanes: Conformational Variations, Circularly Polarized Luminescence, and Organic Electroluminescence. J. Am. Chem. Soc. 2017, 139, 11214–11221. [Google Scholar] [CrossRef]
- Shimada, M.; Yamanoi, Y.; Matsushita, T.; Kondo, T.; Nishibori, E.; Hatakeyama, A.; Sugimoto, K.; Nishihara, H. Optical Properties of Disilane-Bridged Donor-Acceptor Architectures: Strong Effect of Substituents on Fluorescence and Nonline-ar Optical Properties. J. Am. Chem. Soc. 2015, 137, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, H.; Hattori, Y.; Yamanoi, Y.; Nishihara, H. Structures and optical properties of tris(trimethylsilyl)silylated oli-gothiophene derivatives. J. Org. Chem. 2014, 79, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Lesbani, A.; Kondo, H.; Sato, J.-I.; Yamanoi, Y.; Nishihara, H. Facile synthesis of hypersilylated aromatic compounds by palladium-mediated arylation reaction. Chem. Commun. 2010, 46, 7784–7786. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Nishihara, H. Unusual reactivity of group 14 hydrides toward organic halides: Synthetic stud-ies and application to functional materials. J. Synth. Org. Chem. Jpn. 2016, 74, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Nakae, M.; Nishio, M.; Yamanoi, Y. Photofunctional Organosilicon Compounds. Bull. Jpn. Soc. Coord. Chem. 2020, 76, 31–39. [Google Scholar] [CrossRef]
- Gualco, P.; Amgoune, A.; Miqueu, K.; Ladeira, S.; Bourissou, D. A Crystalline σ Complex of Copper. J. Am. Chem. Soc. 2011, 133, 4257–4259. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.A.; Press, E.M.; Siegler, M.A.; Klausen, R.S.; Thoi, V.S. 2D Oligosilyl Metal–Organic Frameworks as Multi-state Switchable Materials. Angew. Chem. Int. Ed. 2020, 59, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Lee, H.; Noh, T.H.; Jung, O.-S. In situ crystalline transformation of bis(halo)mercury(ii) coordination polymers to ionic chloro-bridged-bis(halo)mercury(ii) species via UV irradiation in chloroform media. Cryst. Eng. Comm. 2016, 18, 6997–7002. [Google Scholar] [CrossRef]
- Sculfort, S.; Braunstein, P. Intramolecular d10–d10 interactions in heterometallic clusters of the transition metals. Chem. Soc. Rev. 2011, 40, 2741–2760. [Google Scholar] [CrossRef] [Green Version]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef]
- Labrum, N.S.; Chen, C.-H.; Caulton, K.G. A new face for bis(pyrazol-3-yl)pyridine: Incompatible geometric preferences dictates unprecedented pincer ligand connectivity. Inorg. Chim. Acta. 2019, 485, 54–57. [Google Scholar] [CrossRef]
- Starosta, R.; Komarnicka, U.K.; Puchalska, M. Solid state luminescence of CuI and CuNCS complexes with phenanthrolines and a new tris(aminomethyl)phosphine derived from N-methyl-2-phenylethanamine. J. Lumin. 2014, 145, 430–437. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, N.; Hou, H.; Zhu, Y.; Tang, M.; Ng, S.W. Adaptable metal cluster block: Facile construction of photolu-minescent CuI coordination polymeric clusters with metal (I) iodides. J. Mol. Struct. 2007, 827, 195–200. [Google Scholar] [CrossRef]
- Khandar, A.A.; Butcher, R.J.; Abedi, M.; Hosseini-Yazdi, S.A.; Akkurt, M.; Tahir, M.N. Synthesis, characterization and crystal structures of dinuclear macrocyclic Schiff base copper(I) complexes bearing different bridges. Polyhedron 2010, 29, 3178–3182. [Google Scholar] [CrossRef]
- Nishio, M.; Shimada, M.; Omoto, K.; Nakae, T.; Maeda, H.; Miyachi, M.; Yamanoi, Y.; Nishibori, E.; Nakayama, N.; Goto, H.; et al. Selective formation of SHG active and inactive 1,1,2,2-tetramethyl-1-(4-(N,N-dimethylamino)phenyl)-2-(2′-cyanophenyl)disilane crystals under weak external stimuli. J. Phys. Chem. C 2020, 124, 17450–17458. [Google Scholar] [CrossRef]
- Hong, X.-J.; Liu, X.; Zhang, J.-B.; Lin, C.-L.; Wu, X.; Ou, Y.-J.; Yang, J.; Jin, H.-G.; Cai, Y.-P. Two low-dimensional Schiff base copper(i/ii) complexes: Synthesis, characterization and catalytic activity for degradation of organic dyes. Cryst. Eng. Comm. 2014, 16, 7926–7932. [Google Scholar] [CrossRef]
- Wheaton, A.M.; Streep, M.E.; Ohlhaver, C.M.; Nicholas, A.D.; Barnes, F.H.; Patterson, H.H.; Pike, R.D. Alkyl Pyridinium Iodocuprate(I) Clusters: Structural Types and Charge Transfer Behavior. ACS Omega 2018, 3, 15281–15292. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-N.; Zhao, R.-Y.; Xu, H.; Wang, Z.-H.; Liu, Q.-S.; Shahid, M.Z.; Miao, J.-L.; Chen, G.; Li, C. The structures, water stabilities and photoluminescence properties of two types of iodocuprate(i)-based hybrids. Dalton Trans. 2018, 47, 2306–2317. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Jiang, L.; Sun, B.; Young, D.J.; Hor, T.S.A. Five Cu(i) and Zn(ii) clusters and coordination polymers of 2-pyridyl-1,2,3-triazoles: Synthesis, structures and luminescence properties. Cryst. Eng. Comm. 2015, 17, 3305–3311. [Google Scholar] [CrossRef]
- Letko, C.S.; Rauchfuss, T.B.; Zhou, X.; Gray, D.L. Influence of Second Coordination Sphere Hydroxyl Groups on the Reactivity of Copper(I) Complexes. Inorg. Chem. 2012, 51, 4511–4520. [Google Scholar] [CrossRef]
- Díez-Viñuela, J.S.; Gamasa, M.P.; Panera, M. Tetra-, Di-, and Mononuclear Copper(I) Complexes Containing (S,S)-iPr-pybox and (R,R)-Ph-pybox Ligands. Inorg. Chem. 2006, 45, 10043–10045. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Jiang, L.; Young, D.J.; Hor, T.S.A. Luminescent [Cu4I4] aggregates and [Cu3I3]-cyclic coordination polymers supported by quinolyl-triazoles. Dalton Trans. 2015, 44, 6075–6081. [Google Scholar] [CrossRef] [PubMed]
- Sarkara, M.; Pandeya, P.; Bera, J.K. Chiral 1,8-naphthyridine based ligands: Syntheses and characterization of Di- and tetranuclear copper (I) and silver (I) complexes. Inorg. Chim. Acta. 2019, 486, 518–528. [Google Scholar] [CrossRef]
- Kirst, C.; Reichel, M.; Karaghiosoff, K. Coordination complexes of di(2-pyridyl)ketone with copper(I) and their formation in solution and under solvent-free conditions. Inorg. Chim. Acta. 2021, 514, 119951. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wei, G.Z.; Banerjee, D.; Hu, Z.; Li, J. Systematic Approach in Designing Rare-Earth-Free Hybrid Sem-iconductor Phosphors for General Lighting Applications. J. Am. Chem. Soc. 2014, 136, 14230–14236. [Google Scholar] [CrossRef]
- Artem′ev, A.V.; Ryzhikov, M.R.; Taidakov, I.V.; Mariana, I.; Rakhmanova, M.I.; Varaksina, E.A.; Bagryanskaya, I.Y.; Malyshev, S.F.; Belogorlov, N.A. Bright green-to-yellow emitting Cu(I) complexes based on bis(2-pyridyl)phosphine ox-ides: Synthesis, structure and effective thermally activated-delayed fluorescence. Dalton Trans. 2018, 47, 2701–2710. [Google Scholar] [CrossRef]
- Cariati, E.; Bu, X.; Ford, P.C. Solvent- and Vapor-Induced Isomerization between the Luminescent Solids [CuI(4-pic)]4 and [CuI(4-pic)]∞ (pic = methylpyridine). The Structural Basis for the Observed Luminescence Vapochromism. Chem. Mater. 2000, 12, 3385–3391. [Google Scholar] [CrossRef]
- Shimizu, M.; Oda, K.; Bando, T.; Hiyama, T. Preparation, Structure, and Properties of Tris(trimethylsilyl)silyl-substituted Anthracenes: Realization of Ideal Conformation for σ–π Conjugation Involving Eclipse of Si–Si σ-Bond with p-Orbital of Aromatic Ring. Chem. Lett. 2006, 35, 1022–1023. [Google Scholar] [CrossRef]
- Imae, I.; Minami, T.; Kawakami, Y. Electrochemical properties and estimation of HOMO and LUMO levels of permethyl-ated oligosilanes with well-defined structures. Des. Monomers Polym. 2004, 7, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Ohshita, J.; Nodono, M.; Kai, H.; Watanabe, T.; Kunai, A.; Komaguchi, K.; Shiotani, M.; Adachi, A.; Okita, K.; Harima, Y.; et al. Synthesis and Optical, Electrochemical, and Electron-Transporting Properties of Silicon-Bridged Bithiophenes. Organometallics 1999, 18, 1453–1459. [Google Scholar] [CrossRef]
- Kato, M. Luminescent Copper(I) Complexes Exhibiting Chromic Phenomena. Nihon Kessho Gakkaishi 2015, 57, 110–115. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, H.; Wang, D.-X.; Lu, H.-F.; Feng, S.-Y.; Sun, D. Reactant ratio-modulated six new copper(I)–iodide coor-dination complexes based on diverse [CumIm] aggregates and biimidazole linkers: Syntheses, structures and temperature-dependent luminescence properties. Cryst. Eng. Comm. 2013, 15, 7792–7802. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Zhang, X.; Yang, Q.-F.; Huo, Q.-S.; Yu, J.-H.; Jia-Ning Xu, J.-N.; Xu, J.-Q. New copper(I) iodides with bisim-idazole molecules: Synthesis, structural characterization and photoluminescence property. J. Solid State Chem. 2017, 251, 176–185. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Synthesis, Properties and Applications of Organic Dyes and Pigments. Leon 1989, 22, 456. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]





| Complex | 2 | 3 |
|---|---|---|
| λem/nm | 519 | 478 |
| /eV [a] | 0.46 | 0.43 |
| Φ[b] | 0.60 | 0.04 |
| τ/µs | 11 | 2.6 |
| kr /s−1 [c] | 5.5 × 104 | 1.5 × 104 |
| knr /s−1 [d] | 3.6 × 104 | 3.7 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakae, T.; Miyabe, H.; Nishio, M.; Yamada, T.; Yamanoi, Y. Synthesis, Structure, and Photophysical Properties of Yellow-Green and Blue Photoluminescent Dinuclear and Octanuclear Copper(I) Iodide Complexes with a Disilanylene-Bridged Bispyridine Ligand. Molecules 2021, 26, 6852. https://doi.org/10.3390/molecules26226852
Nakae T, Miyabe H, Nishio M, Yamada T, Yamanoi Y. Synthesis, Structure, and Photophysical Properties of Yellow-Green and Blue Photoluminescent Dinuclear and Octanuclear Copper(I) Iodide Complexes with a Disilanylene-Bridged Bispyridine Ligand. Molecules. 2021; 26(22):6852. https://doi.org/10.3390/molecules26226852
Chicago/Turabian StyleNakae, Toyotaka, Hiroto Miyabe, Masaki Nishio, Teppei Yamada, and Yoshinori Yamanoi. 2021. "Synthesis, Structure, and Photophysical Properties of Yellow-Green and Blue Photoluminescent Dinuclear and Octanuclear Copper(I) Iodide Complexes with a Disilanylene-Bridged Bispyridine Ligand" Molecules 26, no. 22: 6852. https://doi.org/10.3390/molecules26226852
APA StyleNakae, T., Miyabe, H., Nishio, M., Yamada, T., & Yamanoi, Y. (2021). Synthesis, Structure, and Photophysical Properties of Yellow-Green and Blue Photoluminescent Dinuclear and Octanuclear Copper(I) Iodide Complexes with a Disilanylene-Bridged Bispyridine Ligand. Molecules, 26(22), 6852. https://doi.org/10.3390/molecules26226852