Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis
Abstract
:1. Introduction
2. Association between Gut Microbiota and T2DM
2.1. Alteration of Gut Microbiota Composition with T2DM
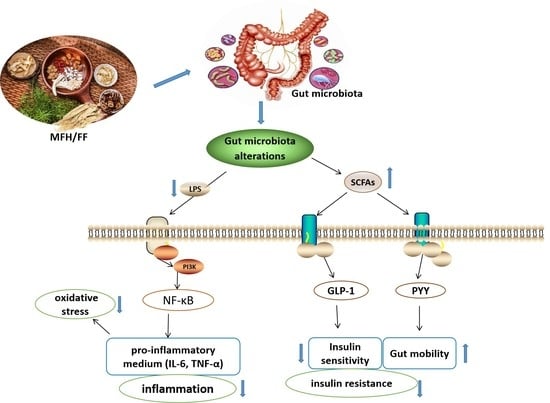
2.2. Mechanism of Gut Microbiota Alteration Causing T2DM
3. Bioactive Ingredients of MFH and FF Target for Microbiota in T2DM
3.1. Saponins
3.2. Polysaccharides
3.3. Flavonoids
3.4. Terpenoids
3.5. Alkaloids
3.6. Others
4. Herb Extracts of MFH and FF Target for Microbiota in T2DM
4.1. Single Herb Extracts of MFH and FF Target for Microbiota in T2DM
4.2. Herb Formula Consisted of MFH for T2DM by Regulating Microbiota
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-beta1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Da Rocha Fernandes, J.; Ogurtsova, K.; Linnenkamp, U.; Guariguata, L.; Seuring, T.; Zhang, P.; Cavan, D.; Makaroff, L.E. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res. Clin. Pract. 2016, 117, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Danda, R.S.; Habiba, N.M.; Rincon-Choles, H.; Bhandari, B.K.; Barnes, J.L.; Abboud, H.E.; Pergola, P.E. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005, 68, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Al-Jameel, S.S. Association of diabetes and microbiota: An update. Saudi J. Biol. Sci. 2021, 28, 4446–4454. [Google Scholar] [CrossRef] [PubMed]
- Needell, J.C.; Zipris, D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr. Diabetes Rep. 2016, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y.; et al. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ji, M.; Xu, J.; Zhang, C.; Li, M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020, 60, 2303–2326. [Google Scholar] [CrossRef]
- Kankanala, J.; Kirby, K.A.; Huber, A.D.; Casey, M.C.; Wilson, D.J.; Sarafianos, S.G.; Wang, Z. Design, synthesis and biological evaluations of N-Hydroxy thienopyrimidine-2,4-diones as inhibitors of HIV reverse transcriptase-associated RNase H. Eur. J. Med. Chem. 2017, 141, 149–161. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Fan, H.; Li, Z.; He, Y.; Liu, C. Safety and therapeutic effects of anti-fibrotic Traditional Chinese Medicine Fuzheng Huayu on persistent advanced stage fibrosis following 2 years entecavir treatment: Study protocol for a single arm clinical objective performance criteria trial. Contemp. Clin. Trials Commun. 2020, 19, 100601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hong, Y.; Lin, X.; Shen, L.; Feng, Y. Recent pharmaceutical evidence on the compatibility rationality of traditional Chinese medicine. J. Ethnopharmacol. 2017, 206, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relman, D.A.; Falkow, S. The meaning and impact of the human genome sequence for microbiology. Trends Microbiol. 2001, 9, 206–214. [Google Scholar] [CrossRef]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M.; Shang, A.; Mao, Q.Q.; Li, H.B. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods 2019, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network Pharmacology Studies on the Bioactive Compounds and Action Mechanisms of Natural Products for the Treatment of Diabetes Mellitus: A Review. Front. Pharmacol. 2017, 8, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.-W.; Hung, W.-C. How gut microbiota relate to the oral antidiabetic treatment of type 2 diabetes. Med. Microecol. 2020, 3, 100007. [Google Scholar] [CrossRef]
- Li, L.J.; Wu, Z.W.; Xiao, D.S.; Sheng, J.F. Changes of gut flora and endotoxin in rats with D-galactosamine-induced acute liver failure. World J. Gastroenterol. 2004, 10, 2087–2090. [Google Scholar] [CrossRef]
- Chen, H.; Yang, H.; Deng, J.; Fan, D. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Regulating of Intestinal Flora and the TLR4/NF-kappaB Signaling Pathway in C57BL/6 Mice. J. Agric. Food Chem. 2021, 69, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Pi, Z.F.; Yue, H.; Yang, H.; Wang, Y.; Yu, Q.; Liu, S.Y. Effect of 20(S)-ginsenoside Rg3 on streptozotocin-induced experimental type 2 diabetic rats: A urinary metabonomics study by rapid-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Yuk, H.G.; Ko, S.G.; Cho, S.G.; Moon, G.S. Gut Microbiome Prolongs an Inhibitory Effect of Korean Red Ginseng on High-Fat-Diet-Induced Mouse Obesity. Nutrients 2021, 13, 926. [Google Scholar] [CrossRef]
- Luo, J.; Chai, Y.; Zhao, M.; Guo, Q.; Bao, Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020, 11, 4327–4338. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Guo, L.-X.; Hu, W.-H.; Peng, Z.-T.; Wang, C.; Chen, Z.-C.; Liu, E.Y.L.; Dong, T.T.X.; Wang, T.-J.; Tsim, K.W.K. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods 2019, 63, 103593–103603. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Wang, Y.; Ruan, K.F.; Feng, Y. Effect of MDG-1 on oral glucose tolerance and intestinal microecological balance in diabetic mice. World Chin. J. Dig. 2011, 19, 2058–2062. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Yuan, M.X.; Lu, W.; Bao, Y.H.; Chai, Y.Y. In Vitro Digestion Properties of Polygonatum sibiricum Polysaccharide and Its Regulatory Action on the Gut Microbiota in T2DM Mice. Mod. Food Sci. Technol. 2021, 37, 8–22. [Google Scholar]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef]
- Wang, C.; Yin, Y.; Cao, X.; Li, X. Effects of Maydis stigma polysaccharide on the intestinal microflora in type-2 diabetes. Pharm. Biol. 2016, 54, 3086–3092. [Google Scholar] [CrossRef] [Green Version]
- Nie, Q.; Hu, J.; Gao, H.; Fan, L.; Chen, H.; Nie, S. Polysaccharide from Plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocol. 2019, 86, 34–42. [Google Scholar] [CrossRef]
- Wang, Q.; Chai, D.D.; Wu, X.H.; Ren, L.W.; Liu, Y.N.; Yu, Z.W. Radix Pseudostellariae polysaccharide attenuates high fat diet induced hepatic insulin resistance in mice. Chin. J. Pathophysiol. 2015, 31, 5–10. [Google Scholar]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Xiong, T.; Nie, S.P.; Xie, M.Y. Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. Carbohydr. Polym. 2018, 201, 624–633. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z.; et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Ahmed, D.; Khan, M.I.; Sharma, M.; Khan, M.F. Novel pentacyclic triterpene isolated from seeds of Euryale Ferox Salisb. ameliorates diabetes in streptozotocin induced diabetic rats. Interdiscip. Toxicol. 2018, 11, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Hu, M.; Huang, Z.; Fang, K.; Wang, D.; Chen, Q.; Li, J.; Yang, D.; Zou, X.; Xu, L.; et al. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front. Pharmacol. 2017, 8, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, X.X.; Zhang, Q.Y.; Su, S.L.; Yan, H.; Zhu, Y.; Shang, E.X.; Qian, D.W.; Duan, J.A. Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice. Biomed. Pharmacother. 2020, 125, 109926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Zhao, L.; Zhou, G.; Li, X. Regulating the gut microbiota and SCFAs in the faeces of T2DM rats should be one of antidiabetic mechanisms of mogrosides in the fruits of Siraitia grosvenorii. J. Ethnopharmacol. 2021, 274, 114033–114044. [Google Scholar] [CrossRef]
- Yuan, H.; Shi, F.; Meng, L.; Wang, W. Effect of sea buckthorn protein on the intestinal microbial community in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018, 107, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006. [Google Scholar] [CrossRef]
- Lira Neto, J.C.G.; Damasceno, M.M.C.; Ciol, M.A.; de Freitas, R.; de Araujo, M.F.M.; Teixeira, C.R.S.; Carvalho, G.C.N.; Lisboa, K.; Marques, R.L.L.; Alencar, A.; et al. Efficacy of Cinnamon as an Adjuvant in Reducing the Glycemic Biomarkers of Type 2 Diabetes Mellitus: A Three-Month, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Am. Coll. Nutr. 2021, 1–9, (online ahead of print). [Google Scholar] [CrossRef]
- Peng, X.C.; Huang, L.Z.; Zhan, H.L.; Wang, C.; Zhang, N.; Liu, L. Effects of essential oil from Cinnamomum Cassia on Clostridia flora IV and Bacteroides in gut of rats. Chin. Tradit. Herb. Drugs 2013, 44, 437–443. [Google Scholar]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1488. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Guo, M.; Feng, Y.; Zheng, H.; Lei, P.; Ma, X.; Han, X.; Guan, H.; Hou, D. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice. Exp. Ther. Med. 2016, 12, 3773–3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, B.; Zhang, Y.; Liu, J.; He, L.S.; Zheng, Y.J.; Lian, F.M. Correction to: Prevention of Type 2 diabetes with the Chinese herbal medicine tianqi capsule: A systematic review and meta-analysis. Diabetes Ther. 2017, 8, 1243–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Lian, F.; Yang, L.; Tong, X. Evaluation of the Chinese Herbal medicine jinlida in Type 2 diabetes patients based on stratification: Results of subgroup analysis from a 12-week trial. J. Diabetes 2018, 10, 112–120. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Lu, B.J.; Zhao, M.H.; Rong, Y.Z.; Chen, R.M. Effect of shengmai injection on vascular endothelial and heart functions in patients with coronary heart disease complicated with diabetes mellitus. Chin. J. Integr. Med. 2008, 14, 281–286. [Google Scholar] [CrossRef]
- Wang, Y.; Choi, H.K.; Brinckmann, J.A.; Jiang, X.; Huang, L. Chemical analysis of Panax quinquefolius (North American ginseng): A review. J. Chromatogr. A 2015, 14, 1426. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563–569. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ye, L.; Fang, C.; Li, J.; Liu, L.; Zhang, W. Identification of changes in volatile organic compounds in Ophiopogonis Radix containing spoiled products in different proportions by headspace-gas chromatography-ion mobility spectrometry. J. Food Biochem. 2021, e13802. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y. Shashen-Maidong Decoction-Mediated IFN-gamma and IL-4 on the Regulation of Th1/Th2 Imbalance in RP Rats. BioMed Res. Int. 2019, 2019, 6012473. [Google Scholar] [PubMed] [Green Version]
- He, Q.; Zhang, T.; Jin, B.; Wu, Y.; Wu, J.; Gao, P.; Wu, S. Exploring the Regulatory Mechanism of Modified Huanglian Maidong Decoction on Type 2 Diabetes Mellitus Biological Network Based on Systematic Pharmacology. Evid. Based Complement. Altern. Med. 2021, 2021, 1768720. [Google Scholar] [CrossRef]
- Mao, D.; Tian, X.Y.; Mao, D.; Hung, S.W.; Wang, C.C.; Lau, C.B.S.; Lee, H.M.; Wong, C.K.; Chow, E.; Ming, X.; et al. A polysaccharide extract from the medicinal plant Maidong inhibits the IKK-NF-kappaB pathway and IL-1beta-induced islet inflammation and increases insulin secretion. J. Biol. Chem. 2020, 295, 12573–12587. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lao, W.; Ji, Q.S.; Yang, Z.H.; Yu, G.C.; Zhong, J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [PubMed]
- Zhao, X.Q.; Guo, S.; Lu, Y.Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.X.; Wang, H.Q.; Zhang, W.H.; Duan, J.A. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Michaelsen, T.E.; Gilje, A.; Samuelsen, A.B.; Hogasen, K.; Paulsen, B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol. 2000, 52, 483–490. [Google Scholar] [CrossRef]
- Ren, H.; Cao, J.; Chen, Y.; Li, G. Current research state and exploitation of Apocynum venetum L. North. Hortic. 2008, 7, 4. [Google Scholar]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.L.; Chen, X.B. Progress in research on hypoglycemic effect of traditional Chinese medicine. Zhejiang J. Integr. Tradit. Chin. West. Med. 2015, 25, 1–3. [Google Scholar]
- Chen, F.; Liu, D.B. Advances in anti-diabetes mechanism of active components in Traditional Chinese Medicine. Acta Chin. Med. Pharmacol. 2012, 40, 1–5. [Google Scholar]
- Zhang, B.; Yue, R.; Chen, Y.; Yang, M.; Huang, X.; Shui, J.; Peng, Y.; Chin, J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2019, 2019, 2634898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Xu, J.; Zhu, H.; Wu, J.; Xu, J.D.; Yan, R.; Li, X.Y.; Liu, H.H.; Duan, S.M.; Wang, Z.; et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016, 6, 22474. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, W.; Yang, C.; Shi, W.; Cao, P.; Long, J.; Xiao, L.; Wu, Y.; Liang, J.; Li, X.; et al. Identification of flavonoids in Plumula nelumbinis and evaluation of their antioxidant properties from different habitats. Ind. Crop. Prod. 2019, 127, 36–45. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wu, J.; Li, J.; Xiao, M.; Yang, Y.; Liu, Z.; Cheng, Y. Plumula Nelumbinis: A review of traditional uses, phytochemistry, pharmacology, pharmacokinetics and safety. J. Ethnopharmacol. 2021, 266, 113429. [Google Scholar] [CrossRef]
- Yuan, H.; Meng, S.; Wang, G.; Gong, Z.; Sun, W.; He, G. Hypoglycemic effect of triterpenoid-rich extracts from Euryale ferox shell on normal and streptozotocin-diabetic mice. Pak. J. Pharm. Sci. 2014, 27, 859–864. [Google Scholar]
- Zhang, T.T.; Jiang, J.G. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin. Investig. Drugs 2012, 21, 1625–1642. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Schiffelers, M. Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J. Control. Release 2017, 247, 7. [Google Scholar]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Gu, J.-F.; Su, S.-L.; Guo, J.-M.; Zhu, Y.; Zhao, M.; Duan, J.-A. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods 2017, 36, 362–374. [Google Scholar] [CrossRef]
- Wang, T.; Sun, J.; Liu, C.; He, Q.; Zhou, X.; Wan, J. Effects of Fried Fructus Aurantii Immaturus with Wheat Bran Decoction on Intestinal Flora in rats with Functional Dyspepsia. Chin. Pharm. J. 2021, 56, 1068–1075. [Google Scholar]
- Zhang, W.-Y.; Zhang, H.-H.; Yu, C.-H.; Fang, J.; Ying, H.-Z. Ethanol extract of Atractylodis macrocephalae Rhizoma ameliorates insulin resistance and gut microbiota in type 2 diabetic db/db mice. J. Funct. Foods 2017, 39, 139–151. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, Y.; Gu, W.; Yang, X.; Yu, J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine 2017, 26, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, M.; Ni, Y.; Jiang, S.; Feng, G.; Sang, S.; Du, G. Alpinia oxyphylla Miq. Extract Prevents Diabetes in Mice by Modulating Gut Microbiota. J. Diabetes Res. 2018, 2018, 4230590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Tao, L.; Chen, X.; Jones, T.J.; Wang, K.; Hu, F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients 2020, 12, 959. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Bose, S.; Shin, N.R.; Song, E.J.; Nam, Y.D.; Kim, H. Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Zhang, X. Structural modulation of gut microbiota reveals Coix seed contributes to weight loss in mice. Appl. Microbiol. Biotechnol. 2019, 103, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hou, M.; Chen, X.; Wang, P.; Ren, J.; Liu, J. Mechanism of Astragali Radix Vesicle-like Nanoparticles for Reducing Blood Glucose in db/db Diabetic Mice by Regulating Gut Microbiota. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 111–118. [Google Scholar]
- Wang, S.; Li, X.Y.; Shen, L. Modulation effects of Dendrobium officinale on gut microbiota of type 2 diabetes model mice. FEMS Microbiol. Lett. 2021, 368, 1–5. [Google Scholar] [CrossRef]
- Wang, H. Effects of Hemp Seed Oil-Water Mixture on Intestinal Microbial and Intestinal Immunity in Mice. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2016. [Google Scholar]
- Xin, H. Effect Study of Yam Gruel on Bifidobacterium in the Gut with Diabetic Patients of Type 2. Master’s Thesis, Fujian University of Traditional Chinese Medicine, Fuzhou, China, 2016. [Google Scholar]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wu, F.; Zhu, J.; Gao, Y.; Wan, H. Effect of Wumeiwan on Intestinal Microflora, Inflammatory Factor and Short Chain Fatty Acids in Type 2 Diabetic Rat. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 8–15. [Google Scholar]
- Hussain, A.; Yadav, M.K.; Bose, S.; Wang, J.H.; Lim, D.; Song, Y.K.; Ko, S.G.; Kim, H. Daesiho-Tang Is an Effective Herbal Formulation in Attenuation of Obesity in Mice through Alteration of Gene Expression and Modulation of Intestinal Microbiota. PLoS ONE 2016, 11, e0165483. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, G.; Deng, Y.; Wang, X.; Chen, Y.C.; Tu, X.Y.; Yu, M.; Sheng, J. Effect of Gegen Qinlian Decoction on LPS, TNF-α, IL-6, and intestinal flora in diabetic KK-Ay mice. Chin. Tradit. Herb. Drugs 2017, 48, 1611–1616. [Google Scholar]
- Liu, C.-Z.; Chen, W.; Wang, M.-X.; Wang, Y.; Chen, L.-Q.; Zhao, F.; Shi, Y.; Liu, H.-J.; Dou, X.-B.; Liu, C.; et al. Dendrobium officinale Kimura et Migo and American ginseng mixture: A Chinese herbal formulation for gut microbiota modulation. Chin. J. Nat. Med. 2020, 18, 446–459. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Sun, M.; Xin, L.; Wang, T.; Wei, L.; Yu, C.; Liu, M.; Ni, Y.; Lu, R.; et al. The Chinese Herbal Formula Shenzhu Tiaopi Granule Results in Metabolic Improvement in Type 2 Diabetic Rats by Modulating the Gut Microbiota. Evid. Based Complement. Altern. Med. 2019, 2019, 6976394. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Yang, R.; Zhang, J.; Wang, Z.; Jia, C.; Zhang, F.; Li, S.; Wang, J.; Murtaza, G.; Xie, H.; et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 2018, 130, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, C.; Li, X.; Ao, M.; Yu, L. Effect of Raw and Salt-processed Herb Pair Anemarrhenae Rhizoma-Phellodendri Chinensis Cortex on Gut Microbiota of Type 2 Diabetic Rats Based on 16S T Sequencing Technique. Pharmacol. Clin. Chin. Med. 2020, 36, 150–156. [Google Scholar]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kim, D.S.; Zhang, T.; Ha, C.; Park, S. Combination of Aronia, Red Ginseng, Shiitake Mushroom and Nattokinase Potentiated Insulin Secretion and Reduced Insulin Resistance with Improving Gut Microbiome Dysbiosis in Insulin Deficient Type 2 Diabetic Rats. Nutrients 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; Liu, C.; Chen, M.; Zou, J.; Zhang, Z.; Cui, X.; Jiang, S.; Shang, E.; Qian, D.; Duan, J. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 303–317. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Source | Microbiota Findings | Mechanism | Study Types and Sequencing Method | Animals | Dose and Duration | Refs. | |
|---|---|---|---|---|---|---|---|---|
| Saponins | Ginsenoside Rk3 | Panax notoginseng | ↑ Lactobacillaceae, Helicobacteraceae, Neococcaceae, Bifidobacteriaceae ↓ Ratio of Firmicute to Bacteroidete; | Inhibit the inflammatory cascade by suppressing the TLR4/NF-κB pathway | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 Mice | 60 mg/kg/day; 8 weeks | [26] |
| 20(S)-ginsenoside Rg3 | Panax ginseng C. A. Meyer | ↑ Bacterial diversity | Improve bacterial diversity | In vivo; principal component analysis | Male Wistar rats | 20 mg/kg/day; 2 weeks | [27] | |
| Ginsenoside Rb1 | Panax ginseng C. A. Meyer | Unclear | Inhibit deglycosylation in the diabetic rats | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 100 mg/kg/day; 72 h | [28] | |
| Saponin-containing Korean red ginseng extracts | Korean red ginseng (Panax ginseng Meyer) | ↑ Parabacteroides, Allistipes, Lactobacillus ↓ Barnesiella, Mucispirillum, Lactococcus, Oscillibacter, Helicobacter | Improve IR and glucose intolerance | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 235 mg/kg/day; 4 weeks | [29] | |
| Saponin extract of Polygonatum sibiricum | Polygonatum sibiricum (Liliaceae) | ↑ Bifidobacteria, Lactobacillus; ↓ Enterobacteriaceae, Enterococcus, C. perfringens | Improve IR | In vivo; Bacteria plate count | ICR male mice | 1.0, 1.5, or 2.0 g/kg/day; 5 weeks | [30] | |
| Polysaccharides | Polysaccharides (MDG-1) from Ophiopogonis Radix | Ophiopogon japonicus (Thunb.) Ker-Gawl. (Liliaceae) | ↑ Lactobacillus, Bifidobacterium; ↓ Escherichia coli, Streptococcus | Improve SCFAs metabolism | In vivo; 16S rRNA Sequencing Analysis | KKay mice | 300 mg/kg/day; 8 weeks | [31,32] |
| Homogeneous polysaccharides from crude Lycium barbarum polysaccharides | Lycium barbarum L. | ↑ Firmicutes/Bacteroides, SCFAs | Regulate SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 50 mg/kg/day; 12 weeks | [33] | |
| Polygonatum sibiricum polysaccharide | Polygonatum sibiricum (Liliaceae) | ↑ Firmicutes, Veillonella, Escherichia-Shigella, Klebsiella; ↓ Proteobacteria, Bacteroides | Regulate bacterial diversity | In vitro; 16S rRNA Sequencing Analysis | / | / | [34] | |
| polysaccharide-rich extracts of A. venetum | Apocynum venetum | ↑ Odoribacter, Anaeroplasma, Parasutterella, Muribaculum; ↓ Enterococcus, Klebsiella, Aerococcus. | Attenuate oxidative stress and SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 J mice | 400 mg/kg/day; 4 weeks | [35] | |
| Maydis stigma polysaccharides | Zea mays subsp. mays | ↑ Lactobacillus and Bacteroides | Restore the intestinal microflora balance | In vivo; 16S rRNA Sequencing Analysis | Male KM mice | 400, 600, 800 mg/kg/day; 5 weeks | [36] | |
| Plantago asiatica L. polysaccharides | Plantago asiatica L. | ↑ Colon bacterial diversity, Bacteroides vulgatus, Lactobacillus fermentum, Prevotella loescheii, Bacteroides vulgates | Increase the levels of SCFAs | In vivo; 16S rRNA Sequencing Analysis | Wistar rats | 100, 200 or 400 mg/kg/day; 5 weeks | [37] | |
| Pseudostellariae Radix | Pseudostellaria heterophylla (Miq.) Pax ex Paxet Hoffm. | ↑ Lactobacillus, Bifidobacterium | Attenuate oxidative stress; suppress inflammatory response | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 J | 500 mg/kg/day; 4 weeks | [38] | |
| Polysaccharides of Lactobacillus plantarum-fermented Momordica charantia | Momordica charantia L. | ↑ Lactococcus laudensis, Prevotella loescheii, diversity of gut microbiota, SCFAs ↓ pH value | Attenuate oxidative stress | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 50, 100 mg/kg/day; 4 weeks | [39] | |
| mulberry fruit polysaccharide | Morus alba L. | ↑ Lactobacillus, Allobaculum, Bacteroides, Akkermansia, SCFA (butyrate, propionate). ↓ Firmicutes, Bacillus, Lactobacillus | Attenuate oxidative stress | In vivo; 16S rRNA Sequencing Analysis | Male db/db mice | 500, 800 mg/kg/day; 8 weeks | [40] | |
| Pumpkin polysaccharide | Cucurbita moschata (Duch. ex Lam.) | ↑ Bacteroidetes, Prevotella, Deltaproteobacteria, Oscillospira, Veillonellaceae, Phascolarctobacterium, Sutterella, Bilophila | Increase SCFAs production | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 1000 mg/kg/day; 4 weeks | [41] | |
| Flavonoids | Baicalein | Oroxylum indicum, Scutellaria baicalensis | ↑ Bacteroides, Bacteroidales S24-7 | Alleviate inflammation and IR | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 50, 150 mg/kg/day; 4 weeks | [42] |
| Terpenoids | 2β-hydroxybetulinic acid 3β-oleiate | Euryale ferox salisb. | Unclear | Reduce blood glucose, regulate dyslipidemia and antioxidant enzymes, protect pancreatic β-cell | In vivo | Male Wistar rats | 60 mg/kg/day; 45 days | [43] |
| Alkaloids | Berberine | Coptidis rhizoma and Berberis vulgaris | ↑ Bacteroidetes, Lactobacillaceae; diversity of the gut microbiota ↓ Proteobacteria, Verrucomicrobia | Alleviate inflammation via NF-κB signaling pathways | In vivo; Real-Time PCR Assay | Male Sprague-Dawley rats | 200 mg/kg/day; 6 weeks | [44,45] |
| Others | total glycoside from R. glutinosa leaves | Rehmannia glutinosa | ↑ Firmicutes, norank_f_Bacteroidales_S24-7_group | Regulate glycolipid, inhibit the expression of α-SMA, TGF-β1, Smad3 and Smad4 in the kidney tissues | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 520 mg/kg/day; 6 weeks | [46] |
| low-polar S. grosvenorii glycosides | Siraitia grosvenorii (Swingle) C. | ↑ Elusimicrobium, Lachnospiraceae_UCG-004 | Increase SCFAs production (acetate, butyrate, and 1β-hydroxycholic acid) | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 20 mg/kg/day; 14 days | [47] | |
| sea buckthorn protein | Hippophae rhamnoides L. | ↑ Bifidobacterium, Lactobacillus, Bacteroides ↓ Clostridium coccoides, PH value; | Increase intestinal microorganism diversity and SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | ICR mice | 50, 100 and 200 mg/kg/day; 30 days | [48] | |
| Long chain of inulin-type fructans | inulin | ↑ Firmicutes/Bacteroidetes ratio; Ruminococcaceae, Lactobacilli | Regulate SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | Female NOD/LtJ mice | 5% diet; 24 weeks | [49] | |
| cinnamon oil | Cortex Cinnamomi | ↑ Bacteroides ↓ Clostridia flora IV | Improve IR | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 0.384 g/kg/day; 30 days | [50,51] | |
| MFH/FF | Source | Microbiota Findings | Mechanism | Test Sections | Study Type and Sequencing Method | Animals | Dose and Duration | Refs. |
|---|---|---|---|---|---|---|---|---|
| Fructus Aurantii Immaturus | Citrus aurantium L. | ↓ Lachnospiraceae NK4A136, Prevo tellaceae UCG-003, Prevotellaceae NK3B31, Lachnospiraceae UCG-008, Ruminiclostridium 9, Ruminococcaceae UCG-014; ↑ Lactobacillus, Alloprevotella, Treponema 2 | Restore the intestinal microflora balance | Water extracts of fried Fructus Aurantii Immaturus with wheat bran decoction | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 9 g/kg/day; 14 d | [83] |
| Atractylodes macrocephala Koidz | Atractylodes macrocephala Koidz (Compositae) | ↑ Bacteroides thetaiotaomicron, Methanobrevibacter smithii | Upregulate GLP-1R, PI3K, PDX-1 expressions, and suppress inflammation (decrease FOXO1, NF-κB p65) | Water extracts of Atractylodis macrocephalae Rhizoma (AMK) | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 100 mg/kg/day; 3 weeks | [84] |
| Anemarrhena asphodeloides | Anemarrhena asphodeloides Bge. | ↑ Blautia coccoides (in vitro) ↓ Proteobacteria, Facklamia, Oligella, and Klebsiella | Suppress the increased oxidative stress and inflammatory activation. | Water extract of A. asphodeloides | In vivo; 16S rRNA Sequencing Analysis | Male SPF Wistar rats | 20, 60, 180 mg/kg/day; 4 weeks. | [85] |
| Lycium barbarum | Lycium barbarum L. | ↑ the ratio of Firmicutes to Bacteroidetes; ↓ Parasutterella, Marvinbryantia, Blautia, Ruminococcus_1, Prevotellaceae_NK3B31_group | Improve liver, kidney, and pancreas injury and regulate metabolic profiles | Water extract of L. barbarum leaf | In vivo; 16S rRNA Sequencing Analysis | (SPF)-grade rat | 1.04, 2.08 g/kg/day; 4 weeks | [66] |
| Alpinia oxyphylla Miq. | Alpinia oxyphylla Miq. (Zingiberaceae) | ↑ Akkermansia; ↓ Helicobacter | Modulate gut microbiota composition | Water extract of Alpinia oxyphylla Miq. | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 100, 300, 500 mg/kg/day; 8 weeks | [86] |
| Chinese propolis | Chinese propolis | ↑ Roseburia, Intestinimonas, Parabacteroides goldsteinii, Parabacteroides distasonis; ↓ Faecalibacterium, Prevotella, Bacteroides vulgatus | Reduce inflammation | Ethanol extract of propolis | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 200, 300 mg/kg/day; 12 weeks | [87,88] |
| Puerariae Radix | Pueraria lobata | ↑ Lactococcus, Ruminococcus | Inhibit obesity and inflammatory-related parameters | 30% ethanol extracts of dried root of P. lobata | In vivo; 16S rRNA Sequencing Analysis | Female C57BL/6 J mice | 400 mg/kg/day; 10 weeks | [89] |
| Mulberry leaf | Morus alba L. | ↑ Bacteroidetes, Proteobacteria; Clostridia | Improve IR | mulberry leaf powder | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley male rats | 20% (w/w) in diet; 13 weeks | [90] |
| Coicis Semen | Coix lacryma-jobi L. var. ma-yuen (Roman.) Stapf | ↑ Lactobacillus, Coprococcus, Akkermansia, Akkermansia muciniphila, Lactobacillus agilis | Improve glucose homeostasis | Coicis Semen power included in diet | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 mice | 0.5 g/100 g; 5 weeks | [91] |
| Astragali Radix | Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) | ↑ ratio of Firmicutes/Bacteroidota; Lactobacillales | Regulate gut microbiota | Astragali Radix decoction vesicle-like nanoparticles extracted by ltracentrifugation; | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 5.3, 10.6, 21.1 g/kg/day; 3 weeks | [92] |
| Dendrobium candidum | Dendrobium candidum Wall Ex Lindl | ↑ Akkermansia, Parabacteroides | Improve glucose intolerance and IR | Dendrobium officinale extract | In vivo; 16S rRNA Sequencing Analysis | T2D mice | 1.0 g/kg/day; 30 days | [93] |
| hemp seed | Cannabis sativa L. | ↑ Bacteroidetes; ↓ Firmicutes | Modulate gut microbiota | hemp seed oil-water mixture | In vivo; 16S rRNA Sequencing Analysis | Female KM mice | 0.2, 0.4 mL; 10 days | [94] |
| Dioscoreae Rhizoma | Dioscorea opposita Thunb. | ↑ Bifidobacterium, Adolescentis, Bifidobacterium infantis | Modulate gut microbiota | yam gruel | In vivo; 16S rRNA Sequencing Analysis | Human patients | 150 g/day; 3 months | [95] |
| MFH and FF | Microbiota Findings | Mechanism | Test Sections | Study Type and Sequencing Method | Animals | Dose and Duration | Ref. |
|---|---|---|---|---|---|---|---|
| Wumeiwan | ↓ Bacteroidetes, Actinobacteria, Bacteroides, Clostridium; ↑ Firmicutes, DeltaProteobacteria, Lactobacillus | Improve SCFA, inhibit inflammatory mediums (TNF-α, IL-10) | Decoction concentrate | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 5, 10, 20 g/kg/day; 4 weeks | [97] |
| Daesiho-Tang | ↑ Bacteroidetes, Bacteroidetes/Firmicutes ratio, Akkermansia Bifidobacterium, Lactobacillus; ↓ Firmicutes | Modulate intestinal microbiota | Water extracts | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 mice | 700 mg/kg/day; 12 weeks | [98] |
| Gegen Qinlian Decoction | ↑ Lactobacillus johnsonii, Stomatobaculum longum strain ACC2, Bacteroides vulgatus | Suppress inflammation: reduce the levels of LPS, TNF-α, IL-6 | Crude drugs | In vivo; 16S rRNA Sequencing Analysis | KK-Ay mice | 4.44, 13.30, 40.00 g/kg/day; 4 weeks | [99] |
| A mixture of D. officinale and American ginseng | ↑ ratio of Bacteroidetes to Firmicutes, Prevotella, Akkermansia; and SCFA-producing bacteria; ↓ S24-7/Rikenella/Escherichia coli. | Decrease inflammation (IL-6 and TNF-α) and oxidative stress; improve intestinal flora balance | Mixture of D. officinale and American ginseng | In vivo; 16S rRNA Sequencing Analysis | Dogs | 160 mg/kg/day; 60 days | [100] |
| Chinese Herbal Formula Shenzhu Tiaopi Granule | ↑ Lactobacillus; ↓ Firmicutes/Bacteroidetes ratio, Bacteroidetes, Allobaculum, Desulfovibrionaceae | Inhibit inflammation, ameliorate IR | Shenzhu Tiaopi Granule | In vivo; 16S rRNA Sequencing Analysis | Male Goto-Kakizaki (GK) | 1000 mg/kg/day; 8 weeks | [101] |
| Qijian Mixture | ↑ Bacteroidetes | Inhibit inflammation and oxidative stress | Qijian Mixture | In vivo; 16S rRNA Sequencing Analysis | Male KKay mice | 1.795, 5.385 g/kg/day; 5 weeks | [102] |
| Anemarrhena asphodeloides Bge.and Phellodendron chinense Schneid | ↓ Bacteroidetes; Bacilli, Lactobacillus ↑ Firmicutes, Proteobacteria; Clostridia, Romboutsia, Bacteroides | Improve intestinal microbiota | Decoction concentrate | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 6.48 g/kg/day; 30 days | [103] |
| Combination of Aronia, Red Ginseng, Shiitake Mushroom and Nattokinase | ↓ Clostridales; ↑ Bacterioidales | Improve IR | Water extracts of the combination | In vivo; 16S rRNA Sequencing Analysis | Sprague Dawley rats | 0.5, 1.0 g/kg/day; 12 weeks | [104] |
| Scutellaria baicalensis Georgi, SR and Coptis chinensis Franch, CR | ↑ SCFAs-producing bacteria: Bacteroidales S24-7 group_norank, Eubacterium nodatum group, Parasutterella, Prevotellaceae UCG-001, Ruminiclostridium, Ruminiclostridium ↓ Secondary bile acid-producing bacteria Escherichia Shigella; | Increase microbially derived SCFAs | Water extracts | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 6.3 g/kg/day; 1 month | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Xiao, J. Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis. Molecules 2021, 26, 6934. https://doi.org/10.3390/molecules26226934
Xia X, Xiao J. Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis. Molecules. 2021; 26(22):6934. https://doi.org/10.3390/molecules26226934
Chicago/Turabian StyleXia, Xiaoyan, and Jiao Xiao. 2021. "Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis" Molecules 26, no. 22: 6934. https://doi.org/10.3390/molecules26226934
APA StyleXia, X., & Xiao, J. (2021). Natural Ingredients from Medicine Food Homology as Chemopreventive Reagents against Type 2 Diabetes Mellitus by Modulating Gut Microbiota Homoeostasis. Molecules, 26(22), 6934. https://doi.org/10.3390/molecules26226934