3.2. Synthetic Methods and Analytic Data of Compounds
3.2.1. General Procedure to Compounds 1a–g
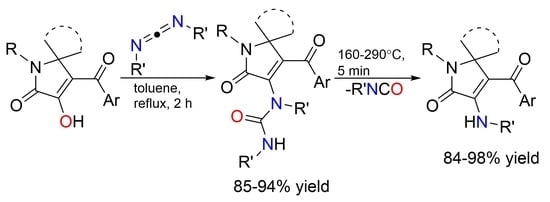
A suspension of the corresponding compound 2 (3.1 mmol) and dicyclohexylurea (3.1 mmol) in 20 mL of toluene was refluxed for 2 h (until the disappearance of the dark violet color of compound 2). Then, the resulting white precipitate was filtered off to afford the desired compound 1.
9-Benzoyl-1,3-dicyclohexyl-8-hydroxy-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1a). Yield: 1.58 g (94%); white solid; mp 285–287 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.90 (s, 1H), 7.75 (m, 2H), 7.62 (m, 1H), 7.51 (m, 2H), 7.24 (m, 1H), 7.00 (m, 1H), 6.91 (m, 1H), 6.81(m, 1H), 3.84 (m, 1H), 3.08 (m, 1H), 2.16–1.96 (m, 2H), 1.87–1.55 (m, 8H), 1.46 (m, 2H), 1.36–1.10 (m, 7H), 0.95 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.5, 169.2, 163.1, 155.5, 154.0, 153.9, 137.3, 132.8, 130.0, 128.6 (2C), 128.2 (2C), 126.7, 120.0, 119.0, 116.7, 113.1, 80.6, 52.1, 51.0, 30.0, 29.5, 28.6 (2C), 25.7, 25.2, 25.2, 25.0, 24.8 (2C) ppm. IR (mineral oil): 3354, 3151, 1779, 1723, 1708, 1678 cm−1. Anal. Calcd (%) for C31H33N3O6: C 68.49; H 6.12; N 7.73. Found: C 68.23; H 6.13; N 7.76. MS (ESI+): m/z calcd for C31H33N3O6+H+: 544.24 [M + H+]; found: 544.18.
1,3-Dicyclohexyl-9-(4-ethoxybenzoyl)-8-hydroxy-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1b). Yield: 1.78 g (98%); white solid; mp 274–276 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.88 (s, 1H), 7.75 (m, 2H), 7.23 (m, 1H), 7.03–6.98 (m, 3H), 6.90 (m, 1H), 6.81 (m, 1H), 4.15 (q, J = 7.0 Hz, 2H), 3.84 (m, 1H), 3.05 (m, 1H), 2.15–1.97 (m, 2H),1.83–1.56 (m, 8H), 1.49–1.41 (m, 2H), 1.37–1.24 (m, 5H), 1.21–1.07 (m, 5H), 0.94 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 186.9, 169.2, 163.2, 162.5, 154.1, 153.9, 131.1 (2C), 130.0, 129.6, 128.1, 126.7, 120.1, 118.9, 116.6, 113.9 (2C), 111.9, 80.7, 63.5, 52.1, 51.0, 30.0, 29.5, 28.7 (2C), 28.6, 25.7, 25.2, 25.0, 24.8 (2C), 14.4 ppm. IR (mineral oil): 3386, 3173, 1777, 1727, 1714, 1683 cm−1. Anal. Calcd (%) for C33H37N3O7: C 67.45; H 6.35; N 7.15. Found: C 67.63; H 6.39; N 7.21. MS (ESI+): m/z calcd for C33H37N3O7+H+: 588.27 [M + H+]; found: 588.24.
1,3-Dicyclohexyl-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-methoxybenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1c). Yield: 1.60 g (90%); white solid; mp 274–276 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.88 (s, 1H), 7.77 (m, 2H), 7.24 (m, 1H), 7.05–6.98 (m, 3H), 6.91 (m, 1H), 6.81 (m, 1H), 3.86–3.81 (m, 4H), 3.06 (m, 1H), 2.15–1.97 (m, 2H), 1.83–1.70 (m, 5H), 1.66–1.54 (m, 3H), 1.49–1.41 (m, 2H), 1.35–1.25 (m, 2H), 1.21–1.08 (m, 5H), 0.94 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 186.9, 169.2, 163.2, 154.1, 153.9, 131.1 (2C), 130.0, 129.8, 128.8, 128.1, 126.7, 120.1, 118.9, 116.6, 113.8, 113.5 (2C), 80.7, 55.4, 52.1, 51.0, 30.0, 29.5, 28.7 (2C), 28.6, 25.7, 25.2, 25.0, 24.8 (2C) ppm. IR (mineral oil): 3385, 3159, 1778, 1727, 1716, 1683 cm−1. Anal. Calcd (%) for C32H35N3O7: C 67.00; H 6.15; N 7.33. Found: C 66.78; H 6.50; N 7.36. MS (ESI+): m/z calcd for C32H35N3O7+H+: 574.26 [M + H+]; found: 574.18.
9-Benzoyl-6-(5-chloro-2-hydroxyphenyl)-1,3-dicyclohexyl-8-hydroxy-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1d). Yield: 1.56 g (87%); white solid; mp 294–296 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.88 (s, 1H), 7.74–7.68 (m, 4H), 7.24 (m, 1H), 6.99 (m, 1H), 6.90 (m, 1H), 6.81 (m, 1H), 3.84 (m, 1H), 3.09 (m, 1H), 2.12–1.98 (m, 2H), 1.84–1.58 (m, 8H), 1.47 (m, 2H), 1.36–1.05 (m, 7H), 0.95 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.5, 169.4, 163.3, 155.7, 153.9, 153.4, 137.3, 132.9, 129.9, 128.7 (2C), 128.3 (2C), 126.3, 121.9, 121.2, 118.2, 113.0, 80.6, 52.2, 51.1, 30.1, 29.5, 29.0, 28.7 (2C), 25.7, 25.3, 25.0, 24.9 (2C) ppm. IR (mineral oil): 3340, 3190, 1782, 1726, 1706, 1672 cm−1. Anal. Calcd (%) for C31H32ClN3O6: C 64.41; H 5.58; N 7.27. Found: C 64.67; H 5.72; N 7.24. MS (ESI+): m/z calcd for C31H32ClN3O6+H+: 578.21 [M + H+]; found: 578.15.
1,3-Dicyclohexyl-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1e). Yield: 1.52 g (88%); white solid; mp 291–293 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.90 (s, 1H), 7.66 (m, 2H), 7.32 (m, 2H), 7.24 (m, 1H), 7.00 (m, 1H), 6.90 (m, 1H), 6.81 (m, 1H), 3.84 (m, 1H), 3.07 (m, 1H), 2.39 (s, 3H), 2.15–1.96 (m, 2H), 1.83–1.54 (m, 8H), 1.47 (m, 2H), 1.36–1.10 (m, 7H), 0.94 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.1, 169.3, 163.2, 154.1, 153.9, 143.4, 134.7, 130.0 (2C), 128.8 (2C), 128.1, 126.7, 120.1, 119.0, 116.7, 113.4, 80.7, 52.1, 51.0, 30.0, 29.5, 28.7 (2C), 28.6, 25.7, 25.3, 25.2, 25.0, 24.8 (2C), 21.1 ppm. IR (mineral oil): 3382, 3182, 1779, 1727, 1715, 1682 cm−1. Anal. Calcd (%) for C32H35N3O6: C 68.92; H 6.33; N 7.54. Found: C 69.11; H 6.38; N 7.57. MS (ESI+): m/z calcd for C32H35N3O6+H+: 558.26 [M + H+]; found: 558.22.
1,3-Dicyclohexyl-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-nitrobenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1f). Yield: 1.70 g (93%); white solid; mp 279–281 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.91 (s, 1H), 8.31 (m, 2H), 7.96 (m, 2H), 7.24 (m, 1H), 7.00 (m, 1H), 6.91 (m, 1H), 6.81 (m, 1H), 3.84 (m, 1H), 3.12 (m, 1H), 2.16–1.95 (m, 2H), 1.89–1.59 (m, 8H), 1.49 (m, 2H), 1.36–1.10 (m, 7H), 0.95 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 186.5, 169.5, 163.2, 158.8, 153.9, 149.4, 142.9, 129.8 (2C), 128.8, 128.1, 126.7, 123.3 (2C), 120.2, 118.9, 116.6, 111.4, 80.4, 52.0, 51.0, 30.0, 29.5, 28.7 (2C), 25.7, 25.2, 25.2, 24.9 (2C), 24.9 ppm. IR (mineral oil): 3381, 3126, 1782, 1727, 1704, 1673 cm−1. Anal. Calcd (%) for C31H32N4O8: C 63.26; H 5.48; N 9.52. Found: C 63.29; H 5.50; N 9.53. MS (ESI+): m/z calcd for C31H32N4O8+H+: 589.23 [M + H+]; found: 589.19.
9-(4-Chlorobenzoyl)-1,3-dicyclohexyl-8-hydroxy-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(1g). Yield: 1.54 g (86%); white solid; mp 284–286 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.89 (s, 1H), 7.77 (m, 2H), 7.58 (m, 2H), 7.24 (m, 1H), 6.99 (m, 1H), 6.90 (m, 1H), 6.81 (m, 1H), 3.84 (m, 1H), 3.08 (m, 1H), 2.15–1.95 (m, 2H), 1.87–1.58 (m, 8H), 1.47 (m, 2H), 1.35–1.05 (m, 7H), 0.94 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 187.2, 169.2, 163.1, 156.3, 154.0, 153.8, 137.6, 136.1, 130.5 (2C), 130.0, 128.4 (2C), 126.7, 120.0, 118.9, 116.6, 112.6, 80.5, 52.0, 51.0, 30.0, 29.5, 28.7 (2C), 25.7, 25.2, 25.2, 24.9, 24.8 (2C) ppm. IR (mineral oil): 3369, 3135, 1781, 1729, 1709, 1658 cm−1. Anal. Calcd (%) for C31H32ClN3O6: C 64.41; H 5.58; N 7.27. Found: C 64.52; H 5.64; N 7.39. MS (ESI+): m/z calcd for C31H32ClN3O6+H+: 578.21 [M + H+]; found: 587.14.
3.2.2. General Procedure to Compounds 4a–n
A suspension of the corresponding compound 1 (1.8 mmol) and the corresponding carbodiimide 5 (1.8 mmol for 5a,c,d; 3.6 mmol for DIC 5b) in 20 mL of toluene was refluxed for 2 h. Then, the resulting precipitate was filtered off to afford the desired compound 4.
9-Benzoyl-1,3-dicyclohexyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)urea(4a). Yield: 1.26 g (93%); white solid; mp 273–275 °C; 1H NMR (400 MHz, CDCl3): δ = 7.85 (m, 2H), 7.62 (m, 1H), 7.47 (m, 2H), 7.19 (m, 1H), 7.01 (m, 2H), 6.88 (m, 1H), 6.73 (br. s, 1H), 4.85 (d, 1H, J = 4.0 Hz), 4.03 (m, 1H), 3.74 (m, 1H), 3.46 (m, 1H), 2.99 (m, 1H), 2.21 (m, 2H), 2.00–1.50 (m, 22H), 1.44–0.97 (m, 16H) ppm; 13C NMR (100 MHz, CDCl3): δ = 189.4, 168.7, 165.8, 154.5, 154.4, 152.7, 142.5, 137.9, 135.8, 134.2, 130.2, 129.0 (2C), 128.7 (2C), 125.6, 121.7, 121.1, 119.4, 82.1, 59.7, 54.6, 52.8, 49.9, 33.3, 33.0, 31.9, 30.4, 30.4, 30.1, 29.1, 29.0, 26.3, 26.1, 26.1, 29.9, 25.8, 25.7, 25.6, 25.3, 25.2, 25.0, 24.9, 24.7 ppm. IR (mineral oil): 3411, 3132, 1781, 1730, 1652 cm−1. Anal. Calcd (%) for C44H55N5O6: C 70.47; H 7.39; N 9.34. Found: C 70.63; H 7.39; N 9.50. MS (ESI+): m/z calcd for C44H55N5O6+H+: 750.42 [M + H+]; found: 750.38.
1-(9-Benzoyl-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-diisopropylurea(4b). Yield: 1.06 g (88%); white solid; mp 231–232 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.96 (s, 1H), 7.85 (m, 2H), 7.68 (m, 1H), 7.52 (m, 2H), 7.23 (m, 1H), 6.99 (m, 1H) 6.91 (m, 1H), 6.82 (m, 1H), 5.78 (d, 1H, J = 4.0 Hz), 4.08 (m, 1H), 3.88 (m, 1H), 3.63 (m, 1H), 3.26 (s, 1H), 3.05 (m, 1H), 2.15–2.03 (m, 2H), 1.81–1.57 (m, 7H), 1.46 (m, 1H), 1.36–1.28 (m, 3H), 1.21–1.11 (m, 5H), 1.06–0.85 (m, 13H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.6, 168.4, 163.9, 154.2, 153.8, 153.6, 143.0, 136.6, 135.6, 134.1, 129.9, 128.6 (4C), 126.6, 120.5, 118.9, 116.6, 81.3, 52.9, 51.5, 49.7, 42.1, 29.8, 29.6, 28.7, 28.5, 25.6, 25.2, 25.1, 25.0, 24.7, 24.5, 22.4, 22.3, 21.4, 19.8 ppm. IR (mineral oil): 3404, 3171, 1771, 1747, 1711, 1671 cm−1. Anal. Calcd (%) for C38H47N5O6: C 68.14; H 7.07; N 10.46. Found: C 67.97; H 7.26; N 10.22. MS (ESI+): m/z calcd for C38H47N5O6+H+: 670.36 [M + H+]; found: 670.30.
1,3-Dicyclohexyl-1-(1,3-dicyclohexyl-9-(4-ethoxybenzoyl)-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)urea(4c). Yield: 1.30 g (91%); white solid; mp 192–194 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.99 (s, 1H), 7.86 (m, 2H), 7.22 (m, 1H), 6.98 (m, 3H), 6.88 (m, 1H), 6.80 (m, 1H) 5.91 (d, 1H, J = 4.0 Hz), 4.20–4.08 (m, 2H), 3.86 (m, 1H), 3.67 (m, 1H), 3.42 (s, 1H), 2.99 (m, 1H), 2.13–2.00 (m, 2H), 1.84–1.45 (m, 18H), 1.38–0.88 (m, 23H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 187.8, 168.3, 164.2, 163.4, 154.3 (2C), 153.6, 142.1, 139.2, 131.5 (2C), 129.9, 128.5, 126.7, 120.7, 118.9, 116.6, 114.3 (2C), 81.4, 63.7, 57.0, 53.0, 51.4, 49.2, 32.6 (2C), 31.3, 30.3, 29.9, 29.6, 28.7, 28.7, 25.6, 25.3, 25.2 (2C), 25.2, 24.9, 24.8, 24.7 (2C), 24.7 (2C), 24.6, 14.33 ppm. IR (mineral oil): 3357, 3175, 1775, 1731, 1716, 1646 cm−1. Anal. Calcd (%) for C46H59N5O7: C 69.58; H 7.49; N 8.82. Found: C 69.71; H 7.45; N 8.89. MS (ESI+): m/z calcd for C46H59N5O7+H+: 794.45 [M + H+]; found: 794.39.
1,3-Dicyclohexyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-9-(4-methoxybenzoyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)urea(4d). Yield: 1.28 g (91%); white solid; mp 230–232 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.96 (s, 1H), 7.88 (m, 2H), 7.22 (m, 1H), 6.98 (m, 3H), 6.89 (m, 1H), 6.81 (m, 1H), 5.87 (d, 1H, J = 8.0 Hz), 3.91–3.84 (m, 4H), 3.67 (m, 1H), 3.43 (m, 1H), 2.99 (m, 1H), 2.11–2.01 (m, 2H), 1.85–1.45 (m, 18H), 1.39–0.86 (m, 20H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 187.8, 168.2, 164.1, 164.0, 154.2 (2C), 153.6, 142.1, 139.1, 131.4 (2C), 129.8, 128.6, 126.6, 120.7, 118.9, 116.5, 113.9 (2C), 81.36, 57.0, 55.6, 53.0, 51.4, 49.2, 32.5 (2C), 32.5, 31.2, 30.2, 29.8, 29.6, 28.7, 28.6, 25.6, 25.3, 25.2 (2C), 25.1, 24.8, 24.7, 24.7, 24.6 (2C), 24.5 ppm. IR (mineral oil): 3341, 3176, 1779, 1732, 1716, 1645 cm−1. Anal. Calcd (%) for C45H57N5O7: C 69.30; H 7.37; N 8.98. Found: C 69.53; H 7.45; N 8.89. MS (ESI+): m/z calcd for C45H57N5O7+H+: 780.43 [M + H+]; found: 780.37.
1-(9-Benzoyl-6-(5-chloro-2-hydroxyphenyl)-1,3-dicyclohexyl-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-dicyclohexylurea(4e). Yield: 1.33 g (94%); white solid; mp 258–260 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.47 (s, 1H), 7.88 (m, 2H), 7.69 (m, 1H), 7.50 (m, 2H), 7.31 (m, 1H), 7.01 (m, 1H) 6.91 (m, 1H), 6.00 (d, 1H, J = 8.0 Hz), 3.89 (m, 1H), 3.63 (m, 1H), 3.53 (m, 1H), 3.01 (m, 1H), 2.15–2.02 (m, 2H), 1.84–1.45 (m, 18H), 1.37–0.83 (m, 20H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.7, 168.3, 164.1, 154.2, 153.6 (2C), 143.1, 137.7, 135.7, 134.5, 129.8, 128.9 (2C), 128.7 (2C), 126.4, 121.9, 121.7, 118.2, 81.3, 57.5, 53.1, 51.6, 49.4, 32.6 (2C), 32.5, 31.3, 30.3, 30.1, 29.7, 29.0, 28.7, 25.7, 25.4 (2C), 25.3 (2C), 25.2, 25.0, 24.9, 24.8 (2C), 24.6 ppm. IR (mineral oil): 3412, 3198, 1782, 1727, 1657 cm−1. Anal. Calcd (%) for C44H54ClN5O6: C 67.37; H 6.94; N 8.93. Found: C 67.43; H 6.88; N 9.02. MS (ESI+): m/z calcd for C44H54ClN5O6+H+: 783.38 [M + H+]; found: 783.32.
1,3-Dicyclohexyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)urea(4f). Yield: 1.22 g (89%); white solid; mp 245–253 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.00 (s, 1H), 7.77 (m, 2H), 7.30 (m, 2H), 7.22 (m, 1H), 6.98 (m, 1H), 6.89 (m, 1H) 6.81 (m, 1H), 5.84 (d, 1H, J = 8.0 Hz), 3.87 (m, 1H), 3.67 (m, 1H), 3.38 (m, 1H), 3.00 (m, 1H), 2.39 (s, 3H), 2.14–2.00 (m, 2H), 1.84–1.45 (m, 18H), 1.41–0.85 (m, 20H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.2, 168.3, 164.0, 154.2, 154.1, 153.6, 145.0, 142.7, 138.3, 133.3 (2C), 129.9, 129.2 (2C), 128.9, 126.7, 120.6, 119.0, 116.6, 81.4, 59.6, 57.2, 53.0, 51.4, 49.2, 32.6, 32.5, 31.2, 30.2, 29.9, 29.6, 28.7, 28.6, 25.6, 25.4, 25.2 (2C), 25.2, 25.1, 24.8, 24.8, 24.7, 24.6 (2C), 21.3 ppm. IR (mineral oil): 3336, 3164, 1778, 1734, 1715, 1650 cm−1. Anal. Calcd (%) for C45H57N5O6: C 70.75; H 7.52; N 9.17. Found: C 70.91; H 7.62; N 9.24. MS (ESI+): m/z calcd for C45H57N5O6+H+: 764.44 [M + H+]; found: 764.34.
1,3-Dicyclohexyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-9-(4-nitrobenzoyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)urea(4g). Yield: 1.27 g (89%); white solid; mp 229–231 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.10 (s, 1H), 8.33 (m, 2H), 8.01 (m, 2H), 7.24 (m, 1H), 6.99 (m, 1H), 6.90 (m, 1H) 6.83 (m, 1H), 5.99 (d, 1H, J = 8.0 Hz), 3.87 (m, 1H), 3.73 (m, 1H), 3.49 (m, 1H), 3.06 (m, 1H), 2.14–1.97 (m, 2H), 1.81–1.41 (m, 22H), 1.36–0.88 (m, 16H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.6, 168.4, 163.8, 154.2, 154.1, 153.6, 150.2, 144.9, 140.6, 138.3, 130.2, 129.9 (2C), 126.8, 123.9 (2C), 120.4, 119.1, 116.7, 81.1, 57.8, 52.9, 51.6, 49.5, 32.5, 32.3 (2C), 29.9, 29.8, 28.7, 28.7, 25.7, 25.6, 25.4, 25.3, 25.2 (2C), 25.0, 24.9 (2C), 24.7, 24.7 (2C), 24.6 ppm. IR (mineral oil): 3336, 3194, 1779, 1731, 1716, 1656 cm−1. Anal. Calcd (%) for C44H54N6O8: C 66.48; H 6.85; N 10.57. Found: C 66.35; H 6.89; N 10.44. MS (ESI+): m/z calcd for C44H54N6O8+H+: 794.40 [M + H+]; found: 794.36.
1-(9-(4-Chlorobenzoyl)-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-dicyclohexylurea(4h). Yield: 1.31 g (93%); white solid; mp 203–204 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.98 (s, 1H), 7.86 (m, 2H), 7.58 (m, 2H), 7.23 (m, 1H), 6.99 (m, 1H), 6.90 (m, 1H), 6.81 (m, 1H), 5.94 (d, 1H, J = 4.0 Hz), 3.87 (m, 1H), 3.73 (m, 1H), 3.33 (m, 1H), 3.03 (m, 1H), 2.14–1.99 (m, 2H), 1.82–1.40 (m, 20H), 1.34–0.87 (m, 18H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.5, 168.3, 163.8, 154.2, 154.0, 153.5, 143.7, 139.0, 134.4, 130.4, 129.9, 128.8 (2C), 126.7, 120.5 (2C), 118.9, 116.6, 81.1, 57.4, 52.9, 51.4, 49.3, 32.5, 32.3 (2C), 31.2, 30.0, 29.8, 29.6, 28.6, 28.6 (2C), 25.6, 25.4, 25.2 (2C), 25.2, 25.1, 25.0, 24.8, 24.6 (2C), 24.6 ppm. IR (mineral oil): 3345, 3181, 1780, 1731, 1718, 1655 cm−1. Anal. Calcd (%) for C44H54ClN5O6: C 67.37; H 6.94; N 8.93. Found: C 67.76; H 7.11; N 9.02. MS (ESI+): m/z calcd for C44H54ClN5O6+H+: 784.38 [M + H+]; found: 784.33. Crystal structure of compound 4h was deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC 2090986.
1-(1,3-Dicyclohexyl-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-diisopropylurea(4i). Yield: 1.05 g (85%); yellow solid; mp 158–160 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.96 (s, 1H), 7.76 (m, 2H), 7.32 (m, 2H), 7.23 (m, 1H), 6.99 (m, 1H) 6.90 (m, 1H), 6.82 (m, 1H), 5.73 (d, 1H, J = 8.0 Hz), 4.08 (m, 1H), 3.87 (m, 1H), 3.64 (m, 1H), 3.26 (s, 1H), 3.03 (m, 1H), 2.39 (s, 3H), 2.14–2.03 (m, 2H), 1.85–1.53 (m, 7H), 1.47 (m, 1H), 1.37–1.28 (m, 3H), 1.20–1.11 (m, 5H), 1.04–0.86 (m, 13H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.1, 168.4, 164.0, 154.1, 153.8, 153.6, 144.8, 142.5, 139.7, 133.1, 130.0, 129.2 (2C), 128.8 (2C), 126.5, 120.6, 118.9, 116.5, 81.3, 52.9, 51.5, 49.6, 42.1, 29.8, 29.6, 28.7, 28.5, 25.6, 25.2, 25.1, 25.0, 24.7, 24.5, 22.4, 22.3, 21.4, 21.17, 19.8 ppm. IR (mineral oil): 3324, 3180, 1799, 1748, 1693, 1669, 1644 cm−1. Anal. Calcd (%) for C39H49N5O6: C 68.50; H 7.22; N 10.24. Found: C 68.65; H 7.26; N 10.30. MS (ESI+): m/z calcd for C39H49N5O6+H+: 684.38 [M + H+]; found: 684.34.
1-(1,3-Dicyclohexyl-6-(2-hydroxyphenyl)-9-(4-methoxybenzoyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-diisopropylurea(4j). Yield: 1.08 g (86%); white solid; mp 150–152 °C; 1H NMR (400 MHz, CDCl3): δ = 7.86 (m, 2H), 7.18 (m, 1H), 7.01 (m, 3H), 6.94 (m, 2H), 6.86 (m, 1H), 4.88 (d, 1H, J = 8.0 Hz), 4.22 (m, 1H), 4.02 (m, 1H), 3.88 (s, 3H), 3.76 (m, 1H), 2.97 (m, 1H), 2.27–2.14 (m, 2H), 2.01 (m, 1H), 1.92–1.69 (m, 6H), 1.60 (m, 1H), 1.51–1.47 (m, 2H), 1.44–0.88 (m, 20H) ppm; 13C NMR (100 MHz, CDCl3): δ = 187.5, 168.8, 165.8, 164.7, 154.5, 154.4, 152.8, 141.5, 138.6, 131.6 (2C), 130.2, 128.5, 125.8, 121.6, 120.9, 119.1, 114.1 (2C), 82.3, 55.5, 54.6, 52.8, 51.2, 43.1, 30.4, 30.2, 29.2, 28.9, 26.1, 25.9, 25.9 (2C), 25.2, 25.0, 23.0, 22.6, 22.0, 20.2 ppm. IR (mineral oil): 3421, 3198, 1716, 1686, 1664, 1649 cm−1. Anal. Calcd (%) for C39H49N5O7: C 66.93; H 7.06; N 10.01. Found: C 67.02; H 7.00; N 10.05. MS (ESI+): m/z calcd for C39H49N5O7+H+: 700.37 [M + H+]; found: 700.32.
1-(9-(4-Chlorobenzoyl)-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-diisopropylurea(4k). Yield: 1.12 g (88%); white solid; mp 154–157 °C; 1H NMR (400 MHz, CDCl3): δ = 7.80 (m, 2H), 7.46 (m, 2H), 7.19 (m, 1H), 6.99 (m, 2H), 6.87 (m, 1H), 4.87 (d, 1H, J = 4.0 Hz), 4.20 (m, 1H), 4.02 (m, 1H), 3.72 (m, 1H), 2.96 (m, 1H), 2.20 (m, 2H), 2.00 (m, 1H), 1.92–1.70 (m, 6H), 1.63–1.62 (m, 1H), 1.51–1.49 (m, 2H), 1.44–1.23 (m, 6H), 1.18–1.00 (m, 14H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.1, 168.7, 165.4, 154.5, 154.3, 152.8, 142.7, 141.0, 137.2, 134.0, 130.3 (3C), 129.2 (2C), 125.7, 121.3, 121.0, 119.0, 82.1, 54.6, 52.8, 51.3, 43.2, 30.4, 30.3, 29.1, 28.9, 26.1, 25.9, 25.8, 25.2, 25.0, 23.4, 23.0, 22.6, 21.9, 20.2 ppm. IR (mineral oil): 3173, 3083, 1702, 1681, 1666 cm−1. Anal. Calcd (%) for C38H46ClN5O6: C 68.81; H 6.58; N 9.94. Found: C 68.85; H 6.63; N 9.89. MS (ESI+): m/z calcd for C38H46ClN5O6+H+: 704.32 [M + H+]; found: 704.30.
9-Benzoyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-1,3-diphenylurea(4l). Yield: 1.14 g (86%); pale yellow solid; mp 148–150 °C; 1H NMR (400 MHz, CDCl3): δ = 7.69 (m, 2H), 7.55 (m, 1H), 7.39 (m, 2H), 7.27–7.17 (m, 4H), 7.16–7.03 (m, 7H), 6.88 (m, 2H), 6.80–6.67 (m, 3H), 3.97 (m, 1H), 3.12 (m, 1H), 2.14 (m, 2H), 2.96–0.95 (m, 18H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.1, 168.1, 164.5, 154.4, 152.7, 152.3, 144.2, 140.4, 137.1, 136.6, 134.4, 134.1, 130.3, 129.9 (2C), 129.0 (2C), 128.9, 128.7 (2C), 127.0, 125.8, 125.1 (2C), 124.5, 121.9, 121.8, 121.2, 120.0 (2C), 119.8, 82.0, 54.4, 52.7, 30.5, 30.2, 29.0 (2C), 26.1, 26.0, 25.8, 25.7, 25.1, 25.1 ppm. IR (mineral oil): 3169, 1778, 1721, 1647 cm−1. Anal. Calcd (%) for C44H43N5O6: C 71.62; H 5.87; N 9.49. Found: C 71.89; H 5.91; N 9.55. MS (ESI+): m/z calcd for C44H43N5O6+H+: 738.33 [M + H+]; found: 738.26.
1-(1,3-Dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-9-(4-ethoxybenzoyl)-1,3-diphenylurea(4m). Yield: 1.21 g (86%); yellow solid; mp 161–163 °C; 1H NMR (400 MHz, CDCl3): δ = 7.72 (m, 2H), 7.32–7.05 (m, 11H), 6.94–6.85 (m, 3H), 6.78 (m, 2H), 6.71 (m, 1H), 4.09 (q, J = 6.8 Hz, 2H), 3.98 (m, 1H), 3.10 (m, 1H), 2.16 (m, 2H), 1.91–0.99 (m, 21H) ppm; 13C NMR (100 MHz, CDCl3): δ = 186.2, 168.1, 164.6, 164.1, 154.4, 152.6, 152.3, 143.4, 140.4, 137.2, 135.8, 131.4 (2C), 130.2, 129.8 (2C), 129.3, 129.0 (2C), 127.1, 125.7, 125.3 (2C), 124.4, 122.0, 121.3, 120.0 (3C), 114.5 (2C), 82.0, 64.0, 54.5, 52.7, 30.5, 30.2, 29.0 (2C), 26.1, 25.9, 25.8 (2C), 25.1 (2C), 14.5 ppm. IR (mineral oil): 3327, 3183, 1778, 1721, 1644 cm−1. Anal. Calcd (%) for C46H47N5O7: C 70.66; H 6.06; N 8.96. Found: C 70.34; H 6.14; N 9.05. MS (ESI+): m/z calcd for C46H47N5O7+H+: 782.36 [M + H+]; found: 782.36.
1,3-Dibutyl-1-(1,3-dicyclohexyl-6-(2-hydroxyphenyl)-2,4,7-trioxo-1,3,6-triazaspiro[4.4]non-8-en-8-yl)-9-(4-ethoxybenzoyl)urea(4n). To precipitate compound 4n, hexane (50 mL) was added to the reaction mixture. Yield: 1.19 g (89%); pale yellow solid; mp 138–140 °C; 1H NMR (400 MHz, CDCl3): δ = 7.71 (m, 2H), 7.20 (m, 1H), 7.03–6.85 (m, 5H), 5.05 (m, 1H), 4.11 (q, J = 6.8 Hz, 2H), 4.05–3.97 (m, 1H), 3.71–3.64 (m, 1H), 3.51–3.44 (m, 1H), 3.05–2.97 (m, 1H), 2.95–2.84 (m, 2H), 2.19 (m, 2H), 2.05–0.87 (m, 35H) ppm; 13C NMR (100 MHz, CDCl3): δ = 187.0, 169.0, 165.4, 164.1, 155.1, 154.4, 152.6, 142.6, 132.4, 131.0 (2C), 130.3, 128.0, 125.5, 121.8, 121.2, 119.5, 114.7 (2C), 82.1, 63.9, 54.6, 52.8, 48.7, 40.7, 31.4 (2C), 31.3, 30.4, 30.1, 29.1, 28.9, 26.1, 25.9, 25.8, 25.2, 25.0, 20.1, 20.0, 14.6, 13.8 (2C) ppm. IR (mineral oil): 3538, 3271, 1780, 1724, 1642 cm−1. Anal. Calcd (%) for C42H55N5O7: C 67.99; H 7.47; N 9.44. Found: C 67.64; H 7.53; N 9.31. MS (ESI+): m/z calcd for C42H55N5O7+H+: 742.42 [M + H+]; found: 742.37.
3.2.3. General Procedure to Compounds 3a–n
The corresponding compound
4 (0.5 mmol) was put into an oven-dried tube, pressed slightly, and then, it was heated at 160–290 °C (the temperature for each compound is given in
Table 2; caution: R′NCO evolves during the reaction). The reaction mixture was cooled to room temperature and scrubbed with hexane (about 10 mL) to give the appropriate compound
3.
9-Benzoyl-1,3-dicyclohexyl-8-(cyclohexylamino)-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3a). Yield: 300 mg (96%); white solid; mp 215–217 °C; 1H NMR (400 MHz, CDCl3): δ = 7.69 (m, 2H), 7.59 (m, 1H), 7.51 (m, 2H), 7.24 (m, 1H), 7.08 (m, 1H), 7.03 (m, 1H), 6.91 (m, 1H), 6.45 (br. s, 1H), 5.60 (d, 1H, J = 8.0 Hz), 3.99 (m, 1H), 2.84 (m, 1H), 2.22–2.10 (m, 2H), 1.97–1.82 (m, 6H), 1.68–1.53 (m, 7H), 1.45–0.75 (m, 16H) ppm; 13C NMR (100 MHz, CDCl3): δ = 189.7, 170.3, 165.7, 154.7, 152.4, 139.9, 132.7, 130.4, 129.0 (2C), 128.1, 126.1, 122.1, 121.5 (2C), 120.0, 107.1, 83.1, 53.8, 52.3, 33.9, 32.9, 32.4, 30.5, 30.0, 29.1, 29.0, 26.4, 26.0, 25.9, 25.9, 25.2, 25.2, 25.1, 24.5, 24.2 ppm. IR (mineral oil): 3363, 3276, 1767, 1724, 1700 cm−1. Anal. Calcd (%) for C37H44N4O5: C 71.13; H 7.10; N 8.97. Found: C 71.20; H 7.17; N 8.93. MS (ESI+): m/z calcd for C37H44N4O5+H+: 625.34 [M + H+]; found: 625.31.
9-Benzoyl-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-8-(isopropylamino)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3b). Yield: 263 mg (90%); white solid; mp 147–149 °C; 1H NMR (400 MHz, CDCl3): δ = 7.70 (m, 2H), 7.58 (m, 1H), 7.49 (m, 2H), 7.23 (m, 1H), 7.05 (m, 2H), 6.90 (m, 1H), 6.49 (m, 1H), 5.49 (d, 1H, J = 8.0 Hz), 3.98 (m, 1H), 3.29 (m, 1H), 2.84 (m, 1H), 2.22–2.09 (m, 2H), 1.97–1.76 (m, 5H), 1.74–1.66 (m, 3H), 1.58–1.51 (m, 2H), 1.39–1.04 (m, 8H), 0.99–0.94 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ = 189.6, 170.2, 165.6, 154.6, 152.4, 139.6, 132.8, 130.4, 129.0 (2C), 128.0 (2C), 126.0, 122.0, 121.5, 119.9, 107.7, 83.0, 53.8, 52.3, 46.9, 30.5, 30.0, 29.0, 29.0, 26.4, 26.0, 25.9, 25.8, 25.2, 25.2, 22.7, 22.4 ppm. IR (mineral oil): 3354, 3262, 3170, 1726 cm−1. Anal. Calcd (%) for C34H40N4O5: C 69.84; H 6.90; N 9.58. Found: C 69.93; H 6.84; N 9.61. MS (ESI+): m/z calcd for C34H40N4O5+H+: 585.31 [M + H+]; found: 585.30. Crystal structure of compound 3b was deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC 2090985.
1,3-Dicyclohexyl-8-(cyclohexylamino)-9-(4-ethoxybenzoyl)-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3c). Yield: 311 mg (93%); white solid; mp 152–154 °C; 1H NMR (400 MHz, CDCl3): δ = 7.70 (m, 2H), 7.23 (m, 1H), 7.07 (m, 1H), 7.02 (m, 1H), 6.97 (m, 2H), 6.90 (m, 1H), 6.45 (s, 1H), 5.46 (br. s, 1H), 4.17–4.09 (m, 2H), 4.00 (m, 1H), 2.81 (m, 1H), 2.24–2.12 (m, 2H), 1.93–1.80 (m, 7H), 1.68–1.44 (m, 11H), 1.38–0.76 (m, 14H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.6, 170.4, 166.0, 163.0, 154.7, 141.6, 132.2, 130.5 (2C), 130.3, 125.9, 122.2, 121.5 (2C), 120.0, 114.7, 107.8, 83.2, 63.9, 53.8, 52.3, 33.1, 32.4, 30.5, 30.0, 29.1, 29.0, 26.4, 26.0, 25.9, 25.9, 25.2, 25.2, 25.2 (2C), 24.6, 24.3, 14.6 ppm. IR (mineral oil): 3196, 1760, 1718, 1687, 1662 cm−1. Anal. Calcd (%) for C39H48N4O6: C 70.04; H 7.23; N 8.38. Found: C 70.16; H 7.20; N 8.44. MS (ESI+): m/z calcd for C39H48N4O6+H+: 669.37 [M + H+]; found: 669.32. Crystal structure of compounds 3c was deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC 2090984.
1,3-Dicyclohexyl-8-(cyclohexylamino)-6-(2-hydroxyphenyl)-9-(4-methoxybenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3d). Yield: 291 mg (91%); white solid; mp 275–277 °C; 1H NMR (400 MHz, CDCl3): δ = 7.71 (m, 2H), 7.23 (m, 1H), 7.08–6.97 (m, 4H), 6.90 (m, 1H), 6.46 (br. s, 1H), 5.48 (d, 1H, J = 12.0 Hz), 4.00 (m, 1H), 3.89 (s, 3H), 2.81 (m, 1H), 2.18 (m, 2H), 1.91–1.82 (m, 7H), 1.68–1.43 (m, 7H), 1.37–0.75 (m, 15H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.5, 170.4, 166.0, 163.6, 154.7, 152.3, 132.4, 130.5 (2C), 130.3, 125.9, 122.2, 121.5, 120.0, 114.2 (2C), 107.7, 83.0, 55.6, 53.8, 53.7, 52.3, 33.1, 32.4, 30.5, 30.0, 29.1, 29.0, 26.4, 26.0, 25.9, 25.9, 25.2, 25.2, 25.1, 24.6, 24.3 ppm. IR (mineral oil): 3376, 3282, 1764, 1723, 1700 cm−1. Anal. Calcd (%) for C38H46N4O5: C 69.70; H 7.08; N 8.56. Found: C 69.79; H 7.03; N 8.61. MS (ESI+): m/z calcd for C38H46N4O5+H+: 639.35 [M + H+]; found: 639.30.
9-Benzoyl-6-(5-chloro-2-hydroxyphenyl)-1,3-dicyclohexyl-8-(cyclohexylamino)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3e). Yield: 297 mg (90%); white solid; mp 240–242 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.23 (s, 1H), 7.66 (m, 3H), 7.57 (m, 2H), 7.30 (m, 1H), 7.00 (m, 1H), 6.90 (m, 1H), 3.85 (m, 1H), 3.01 (m, 1H), 2.16–2.02 (m, 2H), 1.84–1.72 (m, 5H), 1.66–1.13 (m, 17H), 1.07–0.83 (m, 6H), 0.69 (m, 1H), 0.35 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.6, 170.1, 163.5, 153.9, 153.2, 142.9, 139.8, 132.7, 129.7, 128.9 (2C), 127.9, 125.8, 121.8 (2C), 121.5, 118.2, 104.4, 82.3, 54.4, 51.9, 50.9, 31.4, 30.8, 30.2, 29.4, 28.9, 28.7, 25.7, 25.2, 25.1, 24.9, 24.8 (2C), 24.4, 24.3, 24.2 ppm. IR (mineral oil): 3362, 3280, 1767, 1723, 1700 cm−1. Anal. Calcd (%) for C37H43ClN4O5: C 67.41; H 6.58; N 8.50. Found: C 67.53; H 6.62; N 8.54. MS (ESI+): m/z calcd for C37H43ClN4O5+H+: 659.30 [M + H+]; found: 659.31.
1,3-Dicyclohexyl-8-(cyclohexylamino)-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3f). Yield: 313 mg (98%); white solid; mp 159–190 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.82 (s, 1H), 7.58 (m, 2H), 7.38 (m, 2H), 7.22 (m, 1H), 6.98 (m, 1H), 6.88 (m, 1H), 6.80 (m, 1H), 6.76 (m, 1H), 3.83 (m, 1H), 3.01 (m, 1H), 2.39 (s, 3H), 2.15–1.97 (m, 2H), 1.83–1.72 (m, 5H), 1.66–1.41 (m, 7H), 1.35–0.99 (m, 13H), 0.96–0.84 (m, 2H), 0.68 (m, 1H), 0.39 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.4, 170.2, 163.6, 154.0 (2C), 143.0 (2C), 137.4, 129.8, 129.3 (2C), 127.9 (2C), 126.2, 120.5, 118.8, 116.6, 104.6, 82.4, 54.1, 51.9, 50.8, 31.4, 31.1, 30.0, 29.4, 28.7 (2C), 25.7, 25.3, 25.2, 25.1, 24.8 (2C), 24.5, 24.3, 24.2, 21.0 ppm. IR (mineral oil): 3354, 3277, 1787, 1724, 1700 cm−1. Anal. Calcd (%) for C38H46N4O5: C 71.45; H 7.26; N 8.77. Found: C 71.37; H 7.23; N 8.85. MS (ESI+): m/z calcd for C38H46N4O5+H+: 639.35 [M + H+]; found: 639.30.
1,3-Dicyclohexyl-8-(cyclohexylamino)-6-(2-hydroxyphenyl)-9-(4-nitrobenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3g). Yield: 301 mg (90%); white solid; mp 164–166 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.89 (s, 1H), 8.40 (m, 2H), 7.86 (m, 2H), 7.32 (m, 1H), 7.24 (m, 1H), 6.99 (m, 1H), 6.88 (m, 1H), 6.81 (m, 1H), 3.83 (m, 1H), 3.04 (m, 1H), 2.16–1.95 (m, 2H), 1.81–1.70 (m, 5H), 1.65–1.09 (m, 19H), 1.01–0.74 (m, 4H), 0.34 (br. s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 187.5, 170.1, 163.0, 154.0, 153.9, 149.5, 144.7, 144.3, 130.0, 129.4 (2C), 126.2, 124.2 (2C), 120.3, 118.9, 116.7, 82.2, 55.6, 51.84, 50.89, 31.53, 30.1, 29.5, 28.7 (2C), 25.8, 25.3, 25.2, 24.9, 24.9, 24.8, 24.4 (2C), 24.4, 24.3 ppm. IR (mineral oil): 3313, 3270, 1782, 1727, 1700 cm−1. Anal. Calcd (%) for C37H43N5O7: C 66.35; H 6.47; N 10.46. Found: C 66.44; H 6.53; N 10.58. MS (ESI+): m/z calcd for C37H43N5O7+H+: 670.32 [M + H+]; found: 670.25.
9-(4-Chlorobenzoyl)-1,3-dicyclohexyl-8-(cyclohexylamino)-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3h). Yield: 297 mg (90%); white solid; mp 173–175 °C; 1H NMR (400 MHz, CDCl3): δ = 7.65 (m, 2H), 7.49 (m, 2H), 7.24 (m, 1H), 7.04 (m, 2H), 6.92 (m, 1H), 6.38 (s, 1H), 5.77 (br. s, 1H), 3.97 (m, 1H), 2.81 (m, 1H), 2.21–2.09 (m, 2H), 1.95–1.80 (m, 7H), 1.68–1.46 (m, 9H), 1.36–0.85 (m, 13H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.4, 170.1, 165.5, 154.6, 152.3, 139.2, 138.1, 130.5, 129.5 (2C), 129.3 (2C), 126.0, 122.0, 121.6, 120.0, 106.8, 83.1, 53.8, 52.3, 32.9, 32.4, 32.4, 30.5, 30.1, 29.1, 29.0, 26.4, 26.0, 25.9, 25.9, 25.2, 25.2, 25.1, 24.5, 24.3 ppm. IR (mineral oil): 3379, 3278, 1767, 1724, 1701, 1633 cm−1. Anal. Calcd (%) for C37H43ClN4O5: C 67.41; H 6.58; N 8.50. Found: C 67.53; H 6.61; N 8.47. MS (ESI+): m/z calcd for C37H43ClN4O5+H+: 659.30 [M + H+]; found: 659.24.
1,3-Dicyclohexyl-6-(2-hydroxyphenyl)-8-(isopropylamino)-9-(4-methylbenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3i). Yield: 281 mg (94%); white solid; mp 146–148 °C; 1H NMR (400 MHz, CDCl3): δ = 7.62 (m, 2H), 7.28 (m, 1H), 7.21 (m, 1H), 7.07 (m, 1H), 7.00 (m, 1H), 6.89 (m, 1H), 6.58 (br. s, 1H), 5.43 (d, 1H, J = 12.0 Hz), 3.98 (m, 1H), 3.30 (m, 1H), 2.83 (m, 1H), 2.43 (s, 3H), 2.22–2.10 (m, 2H), 1.95–1.65 (m, 8H), 1.56–1.50 (m, 2H), 1.40–0.88 (m, 15H) ppm; 13C NMR (100 MHz, CDCl3): δ = 189.4, 170.2, 165.8, 154.6, 152.4, 143.7, 136.9, 130.3, 129.6 (2C), 128.3 (2C), 126.0, 122.0, 121.4, 119.8, 108.0, 83.1, 52.3, 46.8, 30.5, 30.0, 29.0 (2C), 26.4, 26.0, 25.9 (2C), 25.9, 25.2, 25.2, 22.7, 22.3, 21.6 ppm. IR (mineral oil): 3197, 1760, 1718, 1687 cm−1. Anal. Calcd (%) for C35H42N4O5: C 70.21; H 7.07; N 9.36. Found: C 70.45; H 7.10; N 9.30. MS (ESI+): m/z calcd for C35H42N4O5+H+: 599.32 [M + H+]; found: 599.32.
1,3-Dicyclohexyl-6-(2-hydroxyphenyl)-8-(isopropylamino)-9-(4-methoxybenzoyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3j). Yield: 280 mg (91%); white solid; mp 148–150 °C; 1H NMR (400 MHz, CDCl3): δ = 7.74 (m, 2H), 7.23 (m, 1H), 7.09 (m, 2H), 7.00 (m, 2H), 6.90 (m, 1H), 6.47 (br. s, 1H), 5.34 (d, 1H, J = 8.0 Hz), 3.99 (m, 1H), 3.83 (s, 3H), 3.27 (m, 1H), 2.80 (m, 1H), 2.22–2.12 (m, 2H), 1.92–1.65 (m, 8H), 1.54–1.49 (m, 2H), 1.40–0.83 (m, 14H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.4, 170.3, 166.0, 163.6, 154.7, 152.3, 142.0, 132.0, 130.6 (2C), 130.3, 125.9, 122.2, 121.5, 120.0, 114.2 (2C), 108.5, 83.2, 55.5, 53.8, 52.3, 47.0, 30.5, 30.0, 29.0 (2C), 26.4, 26.0, 25.9, 25.9, 25.2, 25.2, 22.8, 22.3 ppm. IR (mineral oil): 3183, 3060, 1783, 1760, 1739, 1698 cm−1. Anal. Calcd (%) for C35H42N4O6: C 68.38; H 6.89; N 9.11. Found: C 68.46; H 6.92; N 9.08. MS (ESI+): m/z calcd for C35H42N4O6+H+: 615.32 [M + H+]; found: 615.30.
9-(4-Chlorobenzoyl)-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-8-(isopropylamino)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3k). Yield: 285 mg (92%); white solid; mp 151–153 °C; 1H NMR (400 MHz, CDCl3): δ = 7.67 (m, 2H), 7.47 (m, 2H), 7.23 (m, 1H), 7.06 (m, 1H), 7.01 (m, 1H), 6.90 (m, 1H), 6.45 (br. s, 1H), 5.67 (d, 1H, J = 4.0 Hz), 3.96 (m, 1H), 3.29 (m, 1H), 2.80 (m, 1H), 2.21–2.07 (m, 2H), 1.95–1.66 (m, 7H), 1.59–1.51 (m, 2H), 1.39–0.85 (m, 15H) ppm; 13C NMR (100 MHz, CDCl3): δ = 188.3, 170.0, 165.4, 154.5, 152.3, 139.3, 137.8, 130.5, 129.5 (2C), 129.3 (2C), 125.9, 122.0, 121.5, 119.9, 107.4, 83.0, 53.8, 52.3, 47.22, 30.5, 30.1, 29.0, 29.0, 26.4, 26.0, 25.9, 25.8, 25.2, 25.2, 22.7, 22.3 ppm. IR (mineral oil): 3281, 3080, 1774, 1721, 1704 cm−1. Anal. Calcd (%) for C34H39ClN4O5: C 65.96; H 6.35; N 9.05. Found: C 66.04; H 6.30; N 9.11. MS (ESI+): m/z calcd for C34H39ClN4O5+H+: 619.27 [M + H+]; found: 619.22.
9-Benzoyl-1,3-dicyclohexyl-6-(2-hydroxyphenyl)-8-(phenylamino)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3l). Yield: 285 mg (92%); yellow solid; mp 187–189 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.86 (s, 1H), 9.31 (s, 1H), 7.35 (m, 2H), 7.31–7.23 (m, 2H), 7.17 (m, 2H), 7.02 (m, 1H), 6.97–6.89 (m, 3H), 6.84 (m, 1H), 6.74 (m, 3H), 3.89 (m, 1H), 3.08 (m, 1H), 2.20–2.02 (m, 2H), 1.88–0.85 (m, 18H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 189.7, 169.8, 163.8, 154.1, 153.8, 141.8, 140.7, 138.3, 132.1, 129.9, 128.5 (2C), 128.0, 127.8 (2C), 126.2, 123.6, 121.3 (2C), 120.4, 119.0, 116.8, 113.8, 108.5, 82.3, 52.0, 51.0, 30.0, 29.4, 28.8, 28.7, 25.7, 25.3, 25.2, 24.9, 24.8 (2C) ppm. IR (mineral oil): 3398, 3247, 1768, 1723, 1704 cm−1. Anal. Calcd (%) for C37H38N4O5: C 71.83; H 6.19; N 9.06. Found: C 72.15; H 6.07; N 9.10. MS (ESI+): m/z calcd for C37H38N4O5+H+: 619.29 [M + H+]; found: 619.27.
1,3-Dicyclohexyl-9-(4-ethoxybenzoyl)-6-(2-hydroxyphenyl)-8-(phenylamino)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3m). Yield: 312 mg (94%); yellow solid; mp 187–189 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.85 (s, 1H), 9.20 (s, 1H), 7.34 (m, 2H), 7.24 (m, 1H), 7.01 (m, 1H), 6.93 (m, 3H), 6.83 (m, 1H), 6.75 (m, 3H), 6.66 (m, 2H), 4.09 (q, J = 6.8 Hz, 2H), 3.88 (m, 1H), 3.05 (m, 1H), 2.20–2.02 (m, 2H), 1.88–0.85 (m, 21H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.2, 169.9, 163.9, 161.7, 154.1, 153.8, 140.7, 140.5, 131.1, 130.3 (2C), 129.9, 128.2 (2C), 126.2, 123.4, 121.2 (2C), 120.5, 118.9, 116.7, 113.7 (2C), 109.2, 82.3, 63.2, 52.0, 51.0, 30.0, 29.5, 28.8, 28.7, 25.7, 25.3, 25.2, 25.0, 24.8, 24.8, 14.3 ppm. IR (mineral oil): 3354, 3250, 1771, 1720, 1705 cm−1. Anal. Calcd (%) for C39H42N4O6: C 70.68; H 6.39; N 8.45. Found: C 70.89; H 6.43; N 9.01. MS (ESI+): m/z calcd for C39H42N4O6+H+: 663.32 [M + H+]; found: 663.28.
8-(Butylamino)-1,3-dicyclohexyl-9-(4-ethoxybenzoyl)-6-(2-hydroxyphenyl)-1,3,6-triazaspiro[4.4]non-8-ene-2,4,7-trione(3n). Yield: 270 mg (84%) (purity 90%); white solid; mp 130–132 °C; 1H NMR (400 MHz, DMSO-d6): δ = 9.81 (s, 1H), 7.64 (m, 2H), 7.22 (m, 2H), 7.13–6.77 (m, 10H), 4.16–4.06 (m, 3H), 3.83 (m, 1H), 3.15 (m, 1H), 2.98 (m, 2H), 2.55 (m, 1H), 2.40 (m, 1H), 2.15–1.96 (m, 3H), 1.87–0.67 (m, 18H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 188.4, 170.3, 163.7, 162.1, 154.1, 153.8, 143.9, 132.5, 130.4 (2C), 129.9, 126.2, 120.6, 118.9, 116.7, 114.3 (2C), 105.0, 82.4, 63.5, 51.9, 50.8, 45.5, 40.6, 32.2, 30.5, 30.2, 29.5, 28.8, 25.8, 25.4, 24.9, 19.2, 19.1, 14.4, 13.6, 13.3 ppm. IR (mineral oil): 3325, 3175, 1773, 1718, 1642 cm−1. Anal. Calcd (%) for C37H46N4O6: C 69.14; H 7.21; N 8.72. Found: C 68.89; H 7.25; N 8.71. MS (ESI+): m/z calcd for C37H46N4O6+H+: 643.35 [M + H+]; found: 643.30.