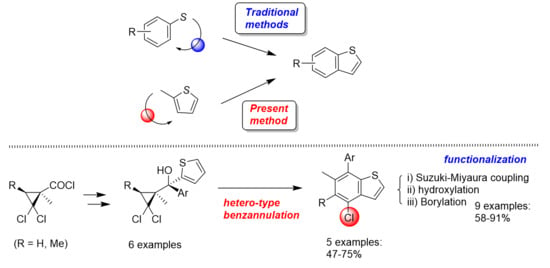
3. Materials and Methods
(
S*)-(2,2-Dichloro-1-methylcyclopropyl)di(thiophen-2-yl)methanol (
3)
![Molecules 26 07008 i001]()
2-Bromothiophene (2.45 g, 15.0 mmol) was added to a stirred suspension of Mg (365 mg, 15.0 mmol) in THF (15 mL) at 20–25 °C under an Ar atmosphere, and the mixture was stirred at the same temperature for 1 h. Methyl (1
S*)-2,2-dichloro-1-methylcyclopropanecarboxylate (commercially available or prepared by the reported method [
9]) (
1; 549 mg, 3.0 mmol) in THF (3.0 mL) was added to the mixture at 0–5 °C, and was stirred at 20–25 °C for 3 h. Sat. NH
4Cl aqueous solution was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na
2SO
4) and concentrated. The obtained crude oil was purified by SiO
2 column chromatography (hexane/AcOEt = 50:1) to give the desired product
3 (739 mg, 77%).
Pale yellow oil; Rf = 0.49 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.35 (d, 1H, J = 7.5 Hz), 1.36 (s, 3H), 2.48 (d, 1H, J = 7.5 Hz), 3.24 (s, 1H), 6.72–6.74 (m, 1H), 6.88–6.91 (m, 1H), 7.06–7.09 (m, 1H), 7.29–7.32 (m, 1H), 7.33–7.35 (m, 1H), 7.40–7.42 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 22.3, 28.7, 39.1, 67.4, 77.2, 125.6, 125.9, 126.2, 126.6, 126.9, 127.3, 146.7, 149.8; IR (neat): νmax = 3545, 3103, 3000, 1663, 1319, 1020, 667 cm−1; HRMS (DART): m/z calcd for C13H12Cl2OS2 [M − OH]+ 300.9679; found: 300.9674.
(
S*)-(2,2-Dichloro-1-methylcyclopropyl)bis(5-methylthiophen-2-yl)methanol (
4)
![Molecules 26 07008 i002]()
nBuLi (1.57 M in hexane, 5.73 mL, 9.0 mmol) was added to a stirred solution of 2-methylthiophene (883 mg, 9.0 mmol) in THF (6.75 mL) at −78 °C under an Ar atmosphere, and the mixture was stirred at the same temperature for 1 h. 2,2-Dichloro-1-methylcyclopropanecarbonyl chloride [
9] (
2; 562 mg, 3.0 mmol) in THF (2.25 mL) was added to the mixture at the same temperature, and gradually warmed up to 20–25 °C for 3 h. Sat. NH
4Cl aqueous solution was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na
2SO
4) and concentrated. The obtained crude oil was purified by SiO
2 column chromatography (hexane/AcOEt = 30:1) to give the desired product
4 (571 mg, 67%).
Pale yellow oil; Rf = 0.65 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.31 (d, 1H, J = 7.5 Hz), 1.36 (s, 3H), 2.43 (d, 1H, J = 7.5 Hz), 2.45 (s, 3H), 2.51 (s, 3H), 3.10 (s, 1H), 6.52–6.56 (m, 2H), 6.68–6.71 (m, 1H), 7.08–7.09 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.4, 22.6, 28.7, 38.8, 67.4, 77.2, 123.7, 124.3, 126.7, 127.2, 140.4, 141.1, 143.9, 147.3; IR (neat): νmax = 3555, 2920, 1449, 1231, 1018, 907 cm−1; HRMS (DART): m/z calcd for C15H16Cl2OS2 [M − OH]+ 328.9992; found: 328.9965.
((1
S*,3
S*)-2,2-Dichloro-1, 3-dimethylcyclopropyl)di(thiophen-2-yl)methanol (
9)
![Molecules 26 07008 i003]()
Following the procedure for the preparation of
4, the reaction using methyl (1
S*,3
S*)-2,2-dichloro-1,3-dimethylcyclopropane-1-carboxylate [
9]
7 (591 mg, 3.0 mmol) derived from methyl angelate,
nBuLi (1.55 M in hexane, 9.68 mL, 15.0 mmol), and thiophene (1.26 g, 15.0 mmol) in THF (18 mL) gave the crude oil, which was purified by SiO
2 column chromatography (hexane/AcOEt = 30:1) to give the desired product
9 (468 mg, 47%).
Pale yellow oil; Rf = 0.35 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.35 (s, 3H), 1.59 (q, 1H, J = 6.9 Hz), 1.73 (d, 3H, J = 6.9 Hz), 3.22 (s, 1H), 6.75–6.77 (m, 1H), 6.89–6.91 (m, 1H), 7.04–7.07 (m, 1H), 7.31–7.34 (m, 2H), 7.38–7.40 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 10.7, 26.3, 37.1, 39.5, 73.0, 80.0, 125.6, 126.0, 126.2, 126.4, 127.0, 127.4, 148.5, 150.0; IR (neat): νmax = 3557, 3107, 2932, 2361, 1450, 1026, 700 cm−1; HRMS (DART): m/z calcd for C14H14Cl2OS2 [M − OH]+ 314.9836; found: 314.9814.
((1
S*,3
R*)-2,2-Dichloro-1, 3-dimethylcyclopropyl)di(thiophen-2-yl)methanol (
10)
![Molecules 26 07008 i004]()
Following the procedure for the preparation of
4, the reaction using methyl (1
S*,3
R*)-2,2-dichloro-1,3-dimethylcyclopropane-1-carboxylate [
9]
8 (985 mg, 5.0 mmol) derived from methyl tiglate,
nBuLi (1.57 M in hexane, 15.9 mL, 25.0 mmol), and thiophene (2.10 g, 25.0 mmol) in THF (30 mL) gave the crude oil, which was purified by SiO
2 column chromatography (hexane/AcOEt = 30:1) to give the desired product
10 (1.13 g, 68%).
Pale yellow oil; Rf = 0.47 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.13 (s, 3H), 1.18 (d, 3H, J = 6.9 Hz), 2.61 (q, 1H, J = 6.9 Hz), 3.25 (s, 1H), 6.70–6.72 (m, 1H), 6.85–6.88 (m, 1H), 7.05–7.08 (m, 1H), 7.28–7.33 (m, 2H), 7.39–7.42 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 9.0, 16.5, 27.4, 40.1, 71.7, 77.7, 125.5, 126.0, 126.2, 126.5, 126.8, 127.3, 146.8, 149.9; IR (neat): νmax = 3547, 3105, 2934, 2361, 1236, 835, 700 cm−1; HRMS (DART): m/z calcd for C14H14Cl2OS2 [M − OH]+ 314.9836; found: 314.9833.
(
S*)-[(
S*)-2,2-Dichloro-1-methylcyclopropyl(phenyl)]methanone [
9] (
12a)
(
S*)-(4-Chlorophenyl)(2,2-dichloro-1-methylcyclopropyl)methanone (
12b)![Molecules 26 07008 i005]()
1-Bromo-4-chlorobenzene (1.15 g, 6.0 mmol) was added to a stirred suspension of Mg (146 mg, 6.0 mmol) in THF (5 mL) at 20–25 °C under Ar atmosphere, and the mixture was stirred at the same temperature for 1 h. Acid chloride 2 (937 mg, 5.0 mmol) in THF (5.0 mL) was added to the mixture at 0–5 °C, which was stirred at 20–25 °C for 3 h. Sat.NH4Cl aqueous solution was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 30:1) to give the desired product 12b (1.06 g, 80%).
Colorless oil; Rf = 0.63 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.50 (d, 1H, J = 7.5 Hz), 1.63 (s, 3H), 2.29 (d, 1H, J = 7.5 Hz), 7.49–7.55 (m, 2H), 7.87–7.92 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 20.6, 30.0, 39.6, 62.2, 129.1 (2C), 131.0 (2C), 132.8, 139.9, 194.3; IR (neat): νmax = 3090, 2936, 1684, 1587, 1091, 986, 773 cm−1; HRMS (DART): m/z calcd for C11H9Cl3O [M + H]+ 262.9797; found: 262.9790.
(
S*)-[(
S*)-2,2-Dichloro-1-methylcyclopropyl)(phenyl)(thiophen-2-yl)]methanol (
13a)
![Molecules 26 07008 i006]()
nBuLi (1.55 M in hexane, 6.45 mL, 10.0 mmol) was added to a stirred solution of thiophen (841 mg, 10.0 mmol) in THF (7.5 mL) at −78 °C under an Ar atmosphere, and the mixture was stirred at the same temperature for 1 h. Ketone 12a (1.15 g, 5.0 mmol) in THF (2.5 mL) was added to the mixture at the same temperature, and gradually warmed up to 20–25 °C for 3 h. Sat. NH4Cl aqueous solution was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 50:1) to give the desired product 13a (813 mg, 52%).
Pale yellow oil; Rf = 0.40 (hexane/AcOEt = 30:1); 1H NMR (500 MHz, CDCl3): δ = 1.29 (s, 3H), 1.32 (d, 1H, J = 7.5 Hz), 2.48 (d, 1H, J = 7.5 Hz), 2.96 (s, 1H), 6.44–6.46 (m, 1H), 6.85–6.88 (m, 1H), 7.28–7.31 (m, 1H), 7.37–7.48 (m, 3H), 7.62–7.66 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 23.0, 28.0, 37.3, 68.0, 79.7, 125.5, 125.6, 126.7, 128.2 (2C), 128.5, 128.9 (2C), 142.0, 150.9; IR (neat): νmax = 3563, 3296, 3088, 2941, 1022, 762, 700 cm−1; HRMS (DART): m/z calcd for C15H14Cl2OS [M − OH]+ 295.0115; found: 295.0109.
(
S*)-(4-Chlorophenyl)((
S*)-2,2-dichloro-1-methylcyclopropyl)(thiophen-2-yl)methanol (
13b)
![Molecules 26 07008 i007]()
Following the procedure for the preparation of 2a, the reaction using ketone 12b (1.05 g, 4.0 mmol), nBuLi (1.55 M in hexane, 5.16 mL, 8.0 mmol), and thiophene (676 mg, 8.0 mmol) in the THF (8.0 mL) gave the crude oil, which was purified by SiO2 column chromatography (hexane/AcOEt = 50:1) to give the desired product 13b (766 mg, 57%).
Pale yellow oil; Rf = 0.53 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.26 (s, 3H), 1.33 (d, 1H, J = 7.5 Hz), 2.64 (d, 1H, J = 7.5 Hz), 2.97 (s, 1H), 6.43–6.45 (m, 1H), 6.86–6.88 (m, 1H), 7.30–7.31 (m, 1H), 7.40–7.44 (m, 2H), 7.55–7.59 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 23.0, 28.0, 37.6, 67.7, 79.3, 125.6, 125.7, 126.7, 128.4 (2C), 130.4 (2C), 134.4, 140.6, 150.4; IR (neat): νmax = 3555, 3075, 3001, 1491, 1094, 1024, 704 cm−1; HRMS (DART): m/z calcd for C15H13Cl3OS [M − OH]+ 328.9725; found: s328.9733.
4-Chloro-6-methyl-7-(thiophen-2-yl)benzo[b]thiophene (
5)
![Molecules 26 07008 i008]()
TiCl4 (1.0 M in 1,2-dichloroethane, 4.1 mL, 4.1 mmol) was added to a solution of alcohol 3 (1.32 g, 4.1 mmol) in 1,2-dichloroethane (83 mL) at 80 °C under an Ar atmosphere, and the mixture was stirred at the same temperature for 0.5 h. After cooling down to room temperature, sat. NaHCO3 aqueous solution was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane) to give the desired product 5 (822 mg, 75%).
Colorless crystals; Rf = 0.34(hexane); mp 67–68 °C; 1H NMR (500 MHz, CDCl3): δ = 2.35 (s, 3H), 7.00–7.02 (m, 1H), 7.13–7.18 (m, 2H), 7.28–7.31 (m, 1H), 7.39–7.41 (m, 1H), 7.44–7.46 (m,1H); 13C NMR (125 MHz, CDCl3): δ = 20.1, 124.7, 126.0, 126.1, 127.1, 127.3, 127.4, 127.7, 127.8, 135.3, 136.4, 139.3, 142.0; IR (neat): νmax = 3105, 2920, 1450, 1231, 826, 696 cm−1; HRMS (DART): m/z calcd for C13H9ClS2 [M + H]+ 264.9912; found: 264.9909.
4-Chloro-2,6-dimethyl-7-(5-methylthiophen-2-yl)benzo[b]thiophene (
6)
![Molecules 26 07008 i009]()
Following the procedure for the preparation of 5, the reaction using alcohol 4 (65 mg, 0.18 mmol) in 1,2-dichloroethane (20 mL) with SnCl4 (1.0 M in dichloromethane, 0.18 mL, 0.18 mmol) in the place of TiCl4, gave the crude oil, which was purified by SiO2 column chromatography (hexane) to give the desired product 6 (28 mg, 53%).
Colorless oil; Rf = 0.77(hexane); 1H NMR (500 MHz, CDCl3): δ = 2.32 (s, 3H), 2.52 (s, 3H), 2.55 (s, 3H), 6.74–6.76 (m, 1H), 6.77–6.80 (m, 1H), 6.83–6.85 (m, 1H), 7.17–7.18 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 15.3, 16.2, 20.2, 122.5, 125.1, 1225.2, 126.5, 127.2, 127.4, 135.1, 135.9, 137.2, 140.3, 142.0, 142.7; IR (neat): νmax = 3063, 2918, 2857, 1574, 1219, 1001, 802 cm−1; HRMS (DART): m/z calcd for C15H13ClS2 [M + H]+ 293.0225; found: 293.0223.
4-Chloro-5,6-dimethyl-7-(thiophen-2-yl)benzo[
b]thiophene (
11)
![Molecules 26 07008 i010]()
Following the procedure for the preparation of 5, the reaction using alcohol 10 (666 mg, 2.0 mmol) and TiCl4 (1.0 M in 1,2-dichloroethane, 2.0 mL, 2.0 mmol) in 1,2-dichloroethane (100 mL) gave the crude oil, which was purified by SiO2 column chromatography (hexane) to give the desired product 11 (266 mg, 48%).
Colorless crystals; Rf = 0.66 (hexane/AcOEt = 30:1); mp 81–82 °C; 1H NMR (500 MHz, CDCl3): δ = 2.29 (s, 3H), 2.52 (s, 3H), 6.97–6.99 (m, 1H), 7.01–7.04 (m, 1H), 7.14–7.17 (m, 1H), 7.29–7.32 (m, 1H), 7.43–7.45 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 17.1, 18.4, 124.8, 125.9, 126.0, 127.0, 127.5, 127.6, 127.7, 131.1, 134.8, 137.0, 139.5, 140.4; IR (neat): νmax = 3017, 2920, 1449, 1323, 1229, 771 cm−1; HRMS (DART): m/z calcd for C14H11ClS2 [M + H]+ 279.0069; found: 279.0054.
4-Chloro-6-methyl-7-phenylbenzo[
b]thiophene (
14a)
![Molecules 26 07008 i011]()
Following the procedure for the preparation of 5, the reaction using alcohol 13a (157 mg, 0.5 mmol) and TiCl4 (1.0 M in 1,2-dichloroethane, 0.5 mL, 0.5 mmol) in 1,2-dichloroethane (5.0 mL) gave the crude oil, which was purified by SiO2 column chromatography (hexane) to give the desired product 14a (61 mg, 47%).
Pale yellow oil; Rf = 0.55(hexane); 1H NMR (500 MHz, CDCl3): δ = 2.25 (s, 3H), 6.93–6.96 (m, 1H), 7.27–7.32 (m, 3H), 7.34–7.43 (m, 2H), 7.44–7.52 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 19.9, 124.6, 126.2, 126.3, 126.9, 127.3, 128.4 (2C), 129.6 (2C), 133.2, 135.5, 136.4, 139.1, 140.9; IR (neat): νmax = 3057, 2920, 1601, 1442, 1364, 907, 700 cm−1; HRMS (DART): m/z calcd for C15H11ClS [M + H]+ 259.0348; found: 259.0361.
4-Chloro-7-(4-chlorophenyl)-6-methylbenzo[
b]thiophene (
14b)
![Molecules 26 07008 i012]()
Following the procedure for the preparation of 5, the reaction using alcohol 13b (174 mg, 0.5 mmol) and TiCl4 (1.0 M in 1,2-dichloroethane, 0.5 mL, 0.5 mmol) in 1,2-dichloroethane (5.0 mL) gave the crude oil, which was purified by SiO2 column chromatography (hexane) to give the desired product 14b (83 mg, 57%).
Colorless oil; Rf = 0.50 (hexane); 1H NMR (500 MHz, CDCl3): δ = 2.24 (s, 3H), 6.91–6.93 (m, 1H), 7.22–7.25 (m, 2H), 7.29–7.31 (m, 1H), 7.37–7.39 (m, 1H), 7.44–7.47 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 19.8, 124.2, 126.2, 127.3, 128.7 (2C), 129.1, 130.6, 131.0 (2C), 133.2, 133.4, 134.1, 137.5, 140.8; IR (neat): νmax = 3103, 2922, 2361, 1558, 1491, 1015, 826 cm−1; HRMS (DART): m/z calcd for C15H10Cl2OS [M + H]+ 292.9959; found: 292.9937.
6-Methyl-4-phenyl-7-(thiophen-2-yl)benzo[
b]thiophene (
15)
![Molecules 26 07008 i013]()
A mixture of 5 (132 mg, 0.50 mmol), PhB(OH)2 (91 mg, 0.75 mmol), K3PO4 (212 mg, 1.00 mmol), Pd(OAc)2 (3.4 mg, 0.015 mmol), and SPhos (12 mg, 0.030 mmol) in toluene (1 mL) was stirred at 80–85 °C for 2 h. After cooling down, water was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane) to give the desired product 15 (139 mg, 91%).
Colorless crystals; Rf = 0.17 (hexane); mp 136–137 °C; 1H NMR (500 MHz, CDCl3): δ = 2.42 (s, 3H), 7.06–7.08 (m, 1H), 7.17–7.22 (m, 2H), 7.31–7.33 (m, 1H), 7.37–7.39 (m, 1H), 7.41–7.47 (m, 2H), 7.49–7.54 (m, 2H), 7.74–7.78 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 20.2, 124.3, 125.8, 126.7, 126.9, 127.0, 127.5, 128.0, 128.1, 128.2 (2C), 128.8 (2C), 134.3, 136.3, 136.4, 140.2, 140.4, 141.5; IR (neat): νmax = 3028, 2922, 2359, 1576, 1443, 1360, 906 cm−1; HRMS (DART): m/z calcd for C19H14S2 [M + H]+ 307.0615; found: 307.0600.
4-(4-Methoxyphenyl)-6-methyl-7-(thiophen-2-yl)benzo[
b]thiophene (
16)
![Molecules 26 07008 i014]()
Following the procedure for the preparation of 15, the reaction of 5 (79 mg, 0.30 mmol) with 4-MeOC6H4B(OH)2 (68 mg, 0.45 mmol), K3PO4 (127 mg, 0.60 mmol), Pd(OAc)2 (2.2 mg, 0.010 mmol), SPhos (8.2 mg, 0.020 mmol) in toluene (1 mL) and the successive purification by SiO2 column chromatography (hexane/AcOEt = 30:1) gave the desired product 16 (85 mg, 82%).
Colorless crystals; Rf = 0.44 (hexane/AcOEt = 10:1); mp 105–106 °C; 1H NMR (500 MHz, CDCl3): δ = 2.41 (s, 3H), 3.89 (s, 3H), 7.03–7.08 (m, 3H), 7.16–7.22 (m, 2H), 7.27–7.30 (m, 1H), 7.36–7.39 (m, 1H), 7.44–7.47 (m, 1H), 7.68–7.71 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 20.2, 55.3, 114.2 (2C), 124.3, 125.7, 126.6, 126.7, 127.0, 127.5, 127.7, 129.3 (2C), 132.9, 134.3, 136.1, 136.3, 140.3, 141.4, 159.4; IR (neat): νmax = 2955, 2359, 1611, 1514, 1246, 1179, 906 cm−1; HRMS (DART): m/z calcd for C20H16O1S2 [M + H]+ 337.0721; found: 337.0706.
6-Methyl-4,7-di(thiophen-2-yl)benzo[
b]thiophene (
17)
![Molecules 26 07008 i015]()
Following the procedure for the preparation of 15, the reaction of 14a (79 mg, 0.30 mmol) with 2-thienylboronic acid (58 mg, 0.45 mmol), K3PO4 (127 mg, 0.60 mmol), Pd(OAc)2 (2.2 mg, 0.010 mmol), SPhos (8.2 mg, 0.020 mmol) in toluene (1 mL) and the successive purification by SiO2 column chromatography (hexane) gave the desired product 17 (62 mg, 63%).
Colorless crystals; Rf = 0.28(hexane); mp 123–124 °C; 1H NMR (500 MHz, CDCl3): δ = 2.40 (s, 3H), 7.04–7.06 (m, 1H), 7.16–7.21 (m, 3H), 7.39–7.42 (m, 2H), 7.43–7.47 (m, 1H), 7.49–7.51 (m, 1H), 7.62–7.64 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 20.2, 124.4, 125.4, 125.5, 125.8, 126.4, 126.7, 127.0, 127.6, 127.8, 128.5, 129.1, 134.2, 135.1, 140.0, 141.8, 142.3; IR (neat): νmax = 3103, 2922, 2359, 2245, 1576, 1456, 906 cm−1; HRMS (DART): m/z calcd for C17H12S3 [M + H]+ 312.0101; found: 312.0091.
5,6-Dimethyl-4-phenyl-7-(thiophen-2-yl)benzo[b]thiophene (
18)
![Molecules 26 07008 i016]()
Following the procedure for the preparation of 15, the reaction of 11 (84 mg, 0.30 mmol) with PhB(OH)2 (55 mg, 0.45 mmol), K3PO4 (127 mg, 0.60 mmol), Pd(OAc)2 (2.2 mg, 0.010 mmol), SPhos (8.2 mg, 0.020 mmol) in toluene (1 mL) and successive purification by SiO2 column chromatography (hexane) gave the desired product 18 (85 mg, 89%).
Colorless crystals; Rf = 0.29 (hexane); mp 143–144 °C; 1H NMR (500 MHz, CDCl3): δ = 2.22 (s, 3H), 2.31 (s, 3H), 7.03–7.08 (m, 2H), 7.15–7.19 (m, 1H), 7.20–7.24 (m, 1H), 7.39–7.46 (m, 4H), 7.48–7.54 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 17.8, 17.9, 124.2, 125.6, 125.8, 126.9, 127.4, 127.7, 128.1, 128.7 (2C), 129.3 (2C), 131.1, 133.8, 136.0, 138.4, 138.8, 140.6, 141.3; IR (neat): νmax = 3069, 2922, 1601, 1441, 1211, 986, 907 cm−1; HRMS (DART): m/z calcd for C20H16S2 [M + H]+ 321.0772; found: 321.0778.
5,6-Dimethyl-4,7-di(thiophen-2-yl)benzo[b]thiophene (
19)
![Molecules 26 07008 i017]()
Following the procedure for the preparation of 15, the reaction of 11 (84 mg, 0.30 mmol) with 2-thienylboronic acid (58 mg, 0.45 mmol), K3PO4 (127 mg, 0.60 mmol), Pd(OAc)2 (2.2 mg, 0.010 mmol), SPhos (8.2 mg, 0.020 mmol) in toluene (1 mL) and successive purification by SiO2 column chromatography (hexane) gave the desired product 19 (81 mg, 82%).
Colorless crystals; Rf = 0.29 (hexane); mp 207–208 °C; 1H NMR (500 MHz, CDCl3): δ = 2.30 (s, 3H), 2.33 (s, 3H), 7.02–7.05 (m, 2H), 7.13–7.15 (m, 1H), 7.16–7.21 (m, 2H), 7.24–7.25 (m, 1H), 7.44–7.46 (m, 1H), 7.47–7.49 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 17.9, 18.0, 124.2, 125.7, 126.0, 126.1, 127.0, 127.2, 127.4, 127.5, 128.5, 129.1, 133.3, 133.8, 138.4, 140.4, 140.8, 141.0; IR (neat): νmax = 3103, 2924, 1798, 1 734, 1433, 1366, 1240, 1207 cm−1; HRMS (DART): m/z calcd for C18H14S3 [M + H]+ 327.0336; found: 327.0337.
6-Methyl-4,7-diphenylbenzo[
b]thiophene (
20)
![Molecules 26 07008 i018]()
Following the procedure for the preparation of 15, the reaction of 14a (104 mg, 0.40 mmol) with PhB(OH)2 (73 mg, 0.60 mmol), K3PO4 (170 mg, 0.80 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol) and SPhos (9.9 mg, 0.024 mmol) in toluene (1 mL), and the successive purification by SiO2 column chromatography (hexane) gave the desired product 20 (81 mg, 68%).
Colorless crystals; Rf = 0.36 (hexane); mp 171–172 °C; 1H NMR (500 MHz, CDCl3): δ = 2.32 (s, 3H), 6.99–7.02 (m, 1H), 7.31–7.34 (m, 2H), 7.35–7.39 (m, 2H), 7.40–7.45 (m, 2H), 7.47–7.54 (m, 4H), 7.76–7.79 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 19.9, 124.2, 126.2, 127.0, 127.1, 127.8, 128.2 (2C), 128.3 (2C), 128. 7 (2C), 129.7 (2C), 132.1, 135.4, 135.9, 136.3, 139.9, 140.3, 140.7; IR (neat): νmax = 3053, 2924, 2357, 1599, 1443, 1358, 1213, 1016 cm−1; HRMS (DART): m/z calcd for C21H16S [M + H]+ 301.1051; found: 301.1053.
4-(4-Methoxyphenyl)-6-methyl-7-phenylbenzo[
b]thiophene (
21)
![Molecules 26 07008 i019]()
Following the procedure for the preparation of 15, the reaction of 14a (78 mg, 0.30 mmol) with 4-MeOC6H4B(OH)2 (68 mg, 0.45 mmol), K3PO4 (127 mg, 0.60 mmol), Pd(OAc)2 (2.2 mg, 0.010 mmol) and SPhos (8.2 mg, 0.020 mmol) in toluene (1 mL), and the successive purification by SiO2 column chromatography (hexane/AcOEt = 30:1) gave the desired product 21 (79 mg, 80%).
Colorless crystals; Rf = 0.56 (hexane/AcOEt = 10:1); mp 155–156 °C; 1H NMR (500 MHz, CDCl3): δ = 2.31 (s, 3H), 3.90 (s, 3H), 6.99–7.01 (m, 1H), 7.04–7.07 (m, 2H), 7.28–7.52 (m, 7H), 7.70–7.73 (m, 2H); 13C NMR (125 MHz, CDCl3): δ = 19.9, 55.3, 114.1 (2C), 124.2, 126.2, 126.8, 127.1, 128.3 (2C), 129.3 (2C), 129.8 (2C), 132.1, 133.1, 135.1, 135.5, 136.3, 140.0, 140.2, 159.3; IR (neat): νmax = 3034, 2930, 2835, 1609, 1502, 1244, 1034 cm−1; HRMS (DART): m/z calcd for C22H18OS [M + H]+ 331.1157; found: 331.1158.
6-Methyl-7-(thiophen-2-yl)benzo[b]thiophen-4-ol (
22)
![Molecules 26 07008 i020]()
A mixture of 5 (140 mg, 0.53 mmol), Pd(dba)2 (5.8 mg, 0.01 mmol), tBu-XPhos (17 mg, 0.04 mmol) and KOH (140 mg, 2.50 mmol) in 1,4-dioxane (0.50 mL) and H2O (0.50 mL) was stirred at 100–105 °C for 14 h. After cooling down, 1M HCl aqueous solution was added to the mixture, which was extracted twice with AcOEt. The organic phase was washed with water, brine, dried (Na2SO4), and concentrated. The obtained crude product was purified by SiO2 column chromatography (hexane/AcOEt = 5:1) to give the desired product 22 (105 mg, 80%).
Colorless crystals; mp 114–115 °C; Rf = 0.34 (hexane/AcOEt = 5:1); 1H NMR (500 MHz, CDCl3): δ = 2.31 (s, 3H), 6.68 (s, 1H), 6.97–6.99 (m, 1H), 7.10–7.12 (m, 1H), 7.13–7.15 (m, 1H), 7.34–7.36 (m, 1H), 7.39–7.42 (m, 1H); 13C NMR (125 MHz, CDCl3): δ = 20.2, 111.4, 121.8, 124.4, 124.7, 125.5, 126.5, 126.9, 127.5, 135.4, 140.4, 143.0, 149.9; IR (neat): νmax = 3491, 3103, 2959, 2338, 1574, 1352, 1242, 1072 cm−1; HRMS (DART): m/z calcd for C13H10OS2 [M + H]+ 247.0251; found: 247.0261.
4,4,5,5-Tetramethyl-2-(6-methyl-7-(thiophen-2-yl)benzo[b]thiophen-4-yl)-1,3,2-dioxaborolane (
23)
![Molecules 26 07008 i021]()
A mixture of 5 (66 mg, 0.25 mmol), bis(pinacolato)diborane (76 mg, 0.30 mmol), NaOAc (31 mg, 0.38 mmol), Pd(dba)2 (6.9 mg, 0.012 mmol), and XPhos (11.9 mg, 0.025 mmol) in 1,4-dioxane (0.50 mL) was heated at 95–100 °C for 14 h. After cooling down, water was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude oil was purified by SiO2 (neutral, Kanto Chemical, 60N) column chromatography (hexane/AcOEt = 30:1) to give the desired product 22 (52 mg, 58%).
Pale yellow crystals; mp 93–94 °C; 0.59 (hexane/AcOEt = 10:1); 1H NMR (500 MHz, CDCl3): δ = 1.42 (s, 12H), 2.37 (s, 3H), 7.02–7.03 (m, 1H), 7.12–7.14 (m, 1H), 7.15–7.17 (m, 1H), 7.38–7.40 (m, 1H), 7.43–7.45 (m, 1H), 7.76 (s, 1H); 13C NMR (125 MHz, CDCl3): δ = 19.9, 24.9 (4C), 84.3 (2C), 123.2, 125.7, 126.5, 126.9, 127.4 (2C), 132.0, 132.7, 134.3, 140.2, 140.4, 143.3; IR (neat): νmax = 3103, 2976, 2926, 1738, 1580, 1371, 1142 cm−1; HRMS (DART): m/z calcd for C19H21BO2S2 [M + H]+ 357.1158; found: 357.1155.