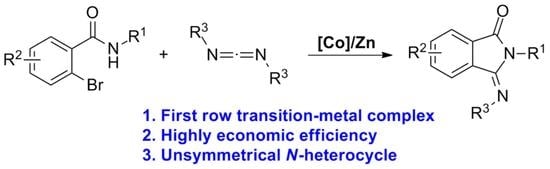
Cobalt-Catalyzed Cyclization of 2-Bromobenzamides with Carbodiimides: A New Route for the Synthesis of 3-(Imino)isoindolin-1-ones
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nan’ya, S.; Tange, T.; Maekawa, E. Synthesis of 1,2-Disubstituted Indoles from o-Phthalaldehyde and Primary Amines. J. Hetrocycl. Chem. 1985, 22, 449–451. [Google Scholar] [CrossRef]
- Murthy, A.R.K.; Wang, O.T.; Reynolds, D.J.; Hall, I.H. Synthesis and Hypolipidemic Activity of 3-Imino-1-Oxoisoindolines in Rodents. Pharm. Res. 1987, 4, 21–27. [Google Scholar] [CrossRef]
- Zhu, X.; Giordano, T.; Yu, Q.-S.; Holloway, H.W.; Perry, T.A.; Lahiri, D.K.; Brossi, A.; Greig, N.H. Thiothalidomides: Novel Isosteric Analogues of Thalidomide with Enhanced TNF-α Inhibitory Activity. J. Med. Chem. 2003, 46, 5222–5229. [Google Scholar] [CrossRef] [PubMed]
- Klíma, J.; Polášek, M.; Ludvík, J.; Urban, J. Reaction of Phthalaldehyde with Aminoethanol under Different Conditions: Products and Mechanisms of Their Formation. J. Hetercycl. Chem. 2012, 49, 1202–1209. [Google Scholar] [CrossRef]
- Miura, T.; Nishida, Y.; Morimoto, M.; Yamauchi, M.; Murakami, M. Palladium-Catalyzed Denitrogenation Reaction of 1,2,3-Benzotriazin-4(3H)-ones Incorporating Isocyanides. Org. Lett. 2011, 13, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Jiang, H.; Yin, M.; Huang, H. Palladium-Catalyzed C−C Coupling of Aryl Halides with Isocyanides: An Alternative Method for the Stereoselective Synthesis of (3E)-(Imino)isoindolin-1-ones and (3E)-(Imino)thiaisoindoline 1,1-Dioxides. Adv. Synth. Catal. 2012, 354, 2288–2300. [Google Scholar] [CrossRef]
- Sueki, S.; Guo, Y.; Kanai, M.; Kuninobu, Y. Rhenium-Catalyzed Synthesis of 3-Imino-1-isoindolinones by C–H Bond Activation: Application to the Synthesis of Polyimide Derivatives. Angew. Chem. Int. Ed. 2013, 52, 11879–11883. [Google Scholar] [CrossRef]
- Tyagi, V.; Khan, S.; Chauhan, P.M.S. A Simple and Efficient Microwave-Assisted Synthesis of Substituted Isoindolinone Derivatives via Ligand-Free Pd-Catalyzed Domino C−C/C−N Coupling Reaction. Synlett 2013, 24, 645–651. [Google Scholar] [CrossRef]
- Hao, W.; Tian, J.; Li, W.; Shi, R.; Huang, Z.; Lei, A. Nickel-Catalyzed Oxidative C−H/N−H Isocyanide Insertion: An Efficient Synthesis of Iminoisoindolinone Derivatives. Chem. Asian J. 2016, 11, 1164–1167. [Google Scholar] [CrossRef]
- Yu, L.; Huang, H.; Chen, X.; Hu, L.; Yu, Y.; Tan, Z. Efficient Synthesis of 3-Hydroxyimino-1-isoindolinones and 3-Methylene-1-isoindolinones via Cu-Promoted C−H Activationnitroalkylation-Intramolecular Cyclization Tandem Processes. Chem. Commun. 2017, 53, 4597–4600. [Google Scholar] [CrossRef]
- Kalsi, D.; Barsu, N.; Dahiya, P.; Sundararaju, B. C−H and N−H Bond Annulation of Benzamides with Isonitriles Catalyzed by Cobalt(III). Synthesis 2017, 49, 3937–3944. [Google Scholar] [CrossRef] [Green Version]
- Kalsi, D.; Barsu, N.; Sundararaju, B. Co(III)-Catalyzed Isonitrile Insertion/Acyl-Group Migration between C–H and N–H Bonds of Arylamides. Chem. Eur. J. 2018, 24, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, L.; Zhou, J.; Jiang, X.; Yu, C. Cobalt-Catalyzed Electrochemical C–H/N–H Functionalization of N-(quinolin-8-yl)benzamide with Isocyanides. Tetrahedron Lett. 2019, 60, 2054–2058. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Wang, J.; Song, G. Multistep Synthesis and Nematicidal Activity of 2-(8-Azabicyclo[3.2.1]octan-3-yl)-3-imino2,3-dihydro-1H-isoindol-1-one Derivatives. Chem. Heterocycl. Compd. 2021, 57, 31–39. [Google Scholar] [CrossRef]
- Liu, C.-C.; Hsieh, J.-C.; Korivi, R.-P.; Cheng, C.-H. Cobalt-Catalyzed Dual Annulation of o-Halobenzaldimine with Alkyne: A Powerful Route toward Bioactive Indenoisoquinolinones. Chem. Eur. J. 2015, 21, 9544–9549. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, D.; Wang, S.; Huang, J. Pd-Catalysed Synthesis of Isoquinolinones and Analogues via C−H and N−H Bonds Double Activation. Chem. Commun. 2012, 48, 3236–3238. [Google Scholar] [CrossRef]
- Obata, A.; Ano, Y.; Chatani, N. Nickel-Catalyzed C–H/N–H Annulation of Aromatic Amides with Alkynes in the Absence of a Specific Chelation System. Chem. Sci. 2017, 8, 6650–6655. [Google Scholar] [CrossRef] [Green Version]
- Hyster, T.K.; Rovis, T. Rhodium-Catalyzed Oxidative Cycloaddition of Benzamides and Alkynes via C–H/N–H Activation. J. Am. Chem. Soc. 2010, 132, 10565–10569. [Google Scholar] [CrossRef] [Green Version]
- Kumon, T.; Wu, J.; Shimada, M.; Yamada, S.; Agou, T.; Fukumoto, H.; Kubota, T.; Hammond, G.B.; Konno, T. Cobalt-Catalyzed C–H Activation/Annulation of Benzamides with Fluorine-Containing Alkynes: A Route to 3- and 4-Fluoroalkylated Isoquinolinones. J. Org. Chem. 2021, 86, 5183–5196. [Google Scholar] [CrossRef]
- Chen, M.-H.; Hsieh, J.-C.; Lee, Y.-H.; Cheng, C.-H. Controlled Synthesis of Enantioselective 1-Aminoindenes via Cobalt-Catalyzed [3 + 2] Annulation Reaction. ACS Catal. 2018, 8, 9364–9369. [Google Scholar] [CrossRef]
- Ackermann, L.; Lygin, A.V.; Hofmann, N. Ruthenium-Catalyzed Oxidative Annulation by Cleavage of C−H/N−H Bonds. Angew. Chem. Int. Ed. 2011, 50, 6379–6382. [Google Scholar] [CrossRef]
- Cera, G.; Haven, T.; Ackermann, L. Iron-Catalyzed C–H/N–H Activation by Triazole Guidance: Versatile Alkyne Annulation. Chem. Commun. 2017, 53, 6460–6463. [Google Scholar] [CrossRef] [PubMed]
- Nohira, I.; Liu, S.; Bai, R.; Lan, Y.; Chatani, N. Nickel-Catalyzed C−F/N−H Annulation of Aromatic Amides with Alkynes: Activation of C–F Bonds under Mild Reaction Conditions. J. Am. Chem. Soc. 2020, 142, 17306–17311. [Google Scholar] [CrossRef] [PubMed]
- Iyori, Y.; Ueno, R.; Morishige, A.; Chatani, N. Nickel-Catalyzed C–O/N–H, C–S/N–H, and C–CN/N–H Annulation of Aromatic Amides with Alkynes: C–O, C–S, and C–CN Activation. Chem. Sci. 2021, 12, 1772–1777. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Chu, Y.-H.; Muralirajan, K.; Cheng, C.-H. A Simple Route to 1,4-Addition Reactions by Co-Catalyzed Reductive Coupling of Organic Tosylates and Triflates with Activated Alkenes. Chem. Commun. 2017, 53, 11584–11587. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Su, H.-L. Synthesis of N-Heterocycles via the Transition-Metal-Catalyzed Tandem Addition/Cyclization of a Nitrile. Synthesis 2020, 52, 819–833. [Google Scholar] [CrossRef]
- Thorat, V.H.; Hsieh, J.-C.; Cheng, C.-H. Transition-Metal-Free Tandem Cyclization/N-Arylation Reaction: A Method to Access Biaryl Sultam Derivatives via a Diradical Pathway. Org. Lett. 2020, 22, 6623–6627. [Google Scholar] [CrossRef]
- Wang, H.-K.; Ciou, Y.-L.; Pallikonda, G.; Wu, H.-L.; Su, H.-L.; Hsieh, J.-C. Copper-Catalyzed Dual Cyclization for the Synthesis of Quinindolines. Molecules 2020, 25, 5303. [Google Scholar] [CrossRef]
- Yeh, L.-H.; Wang, H.-K.; Pallikonda, G.; Ciou, Y.-L.; Hsieh, J.-C. Palladium-Catalyzed Dual Annulation: A Method for the Synthesis of Norneocryptolepine. Org. Lett. 2019, 21, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Jhang, Y.-Y.; Fan-Chiang, T.-T.; Huang, J.-M.; Hsieh, J.-C. Copper-Catalyzed Annulation: A Method for the Systematic Synthesis of Phenanthridinium Bromide. Org. Lett. 2016, 18, 1154–1157. [Google Scholar] [CrossRef]
- Fan-Chiang, T.-T.; Wang, H.-K.; Hsieh, J.-C. Synthesis of Phenanthridine Skeletal Amaryllidaceae Alkaloids. Tetrahedron 2016, 72, 5640–5645. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.-C. Hydride-Induced Anionic Cyclization: An Efficient Method for the Synthesis of 6-H-Phenanthridines via a Transition-Metal-Free Process. Org. Lett. 2015, 17, 1613–1616. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Hsieh, J.-C. Synthesis of Polysubstituted Phenanthridines via Ligand-Free Copper-Catalyzed Annulation. Org. Lett. 2014, 16, 4642–4645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Wu, Y.-S.; Jhan, Y.-H.; Hsieh, J.-C. An Efficient Synthesis of (NH)-Phenanthridinones via Ligand-Free Copper-Catalyzed Annulation. Org. Chem. Front. 2014, 1, 253–257. [Google Scholar] [CrossRef]
- Thorat, V.H.; Aman, H.; Tsai, Y.-L.; Pallikonda, G.; Chuang, G.J.; Hsieh, J.-C. Cobalt-Catalyzed Coupling Reactions of 2-Halobenzamides with Alkynes: Investigation of the Ligand-Controlled Dual Pathways. Org. Chem. Front. 2021, 8, 6419–6426. [Google Scholar] [CrossRef]







| Entry | [Co] | Base | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | Co(dppe)Br2 | Et3N | CH3CN | 47 |
| 2 | Co(dppf)Br2 | Et3N | CH3CN | 26 |
| 3 | Co(dppe)Cl2 | Et3N | CH3CN | 73 |
| 4 | Co(dppf)Cl2 | Et3N | CH3CN | 17 |
| 5 | Co(dppe)Cl2 | pyrolidine | CH3CN | 16 |
| 6 | Co(dppe)Cl2 | pyridine | CH3CN | 21 |
| 7 | Co(dppe)Cl2 | K2CO3 | CH3CN | 0 |
| 8 | Co(dppe)Cl2 | Et3N | CH2Cl2 | trace |
| 9 c | Co(dppe)Cl2 | Et3N | DMF | 13 |
| 10 | Co(dppe)Cl2 | Et3N | THF | 23 |
| 11 | Co(dppe)Cl2 | Et3N | Ethanol | 29 |
| 12 | Co(dppe)Cl2 | Et3N | DMSO | messy |
| 13 | Co(dppe)Cl2 | Et3N | Toluene | 39 |
| 14 c | Co(dppe)Cl2 | Et3N | CH3CN | 72 |
| 15 | CoCl2 | Et3N | CH3CN | 0 |
| 16 d | Co(dppe)Cl2 | Et3N | CH3CN | 0 |
| 17 | - | Et3N | CH3CN | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aman, H.; Huang, Y.-C.; Liu, Y.-H.; Tsai, Y.-L.; Kim, M.; Hsieh, J.-C.; Chuang, G.J. Cobalt-Catalyzed Cyclization of 2-Bromobenzamides with Carbodiimides: A New Route for the Synthesis of 3-(Imino)isoindolin-1-ones. Molecules 2021, 26, 7212. https://doi.org/10.3390/molecules26237212
Aman H, Huang Y-C, Liu Y-H, Tsai Y-L, Kim M, Hsieh J-C, Chuang GJ. Cobalt-Catalyzed Cyclization of 2-Bromobenzamides with Carbodiimides: A New Route for the Synthesis of 3-(Imino)isoindolin-1-ones. Molecules. 2021; 26(23):7212. https://doi.org/10.3390/molecules26237212
Chicago/Turabian StyleAman, Hasil, Yu-Chiao Huang, Yu-Hao Liu, Yu-Lin Tsai, Min Kim, Jen-Chieh Hsieh, and Gary Jing Chuang. 2021. "Cobalt-Catalyzed Cyclization of 2-Bromobenzamides with Carbodiimides: A New Route for the Synthesis of 3-(Imino)isoindolin-1-ones" Molecules 26, no. 23: 7212. https://doi.org/10.3390/molecules26237212
APA StyleAman, H., Huang, Y. -C., Liu, Y. -H., Tsai, Y. -L., Kim, M., Hsieh, J. -C., & Chuang, G. J. (2021). Cobalt-Catalyzed Cyclization of 2-Bromobenzamides with Carbodiimides: A New Route for the Synthesis of 3-(Imino)isoindolin-1-ones. Molecules, 26(23), 7212. https://doi.org/10.3390/molecules26237212