Anti-Fatigue Peptides from the Enzymatic Hydrolysates of Cervus elaphus Blood
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Hydrolysis of DB and Chromatography Fraction of DB Hydrolysates
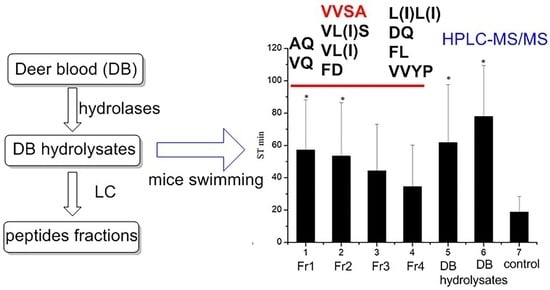
2.2. Anti-Fatigue Evaluation
2.2.1. Effects on Body Weight and Swimming Time
2.2.2. Effects on BUN, HG, and MLA
2.3. Identification of Bioactive Peptides
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials
3.3. Enzymatic Hydrolysis of DB
3.4. Fractioning of Enzymatic Hydrolysates
3.5. HPLC and HPLC-MS Detection
3.6. Experimental Animals and Anti-Fatigue Studies
3.7. Exhaustive Swimming Test
3.8. Biochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| BUN | Blood urea nitrogen |
| DB | Deer blood |
| HG | Hepatic glycogen |
| MLA | Muscle lactic acid |
References
- Serrano, M.P.; Maggiolino, A.; Lorenzo, J.M.; de Palo, P.; Garcia, A.; Landete-Castillejos, T.; Gambin, P.; Cappelli, J.; Dominguez, R.; Perez-Barberia, F.J.; et al. Meat quality of farmed red deer fed a balanced diet: Effects of supplementation with copper bolus on different muscles. Animal 2019, 13, 888–896. [Google Scholar] [CrossRef]
- Ofori, J.A.; Hsieh, Y.H.P. Issues related to the use of blood in food and animal feed. Crit. Rev. Food Sci. Nutr. 2014, 54, 687–697. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.A.; Carne, A.; McConnell, M.A. Slaughterhouse Blood: An Emerging Source of Bioactive Compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Yin, X.P.; Jiang, H.; Gao, X.L.; Le, G.W.; Shi, Y.H. Prepared and separated anti-fatigue peptides from wapiti velvet antler blood protein. Nat. Prod. Res. Dev. 2009, 21, 391–394. [Google Scholar]
- Qi, X.Y.; Zhao, H.P.; Shang, Y.D.; Xu, Y.; Yao, M.J.; Wang, C.F.; Hu, P.F.; Li, C.Y. Deer blood effectively improved clinical signs of anaemia in a rodent model. Anim. Prod. Sci. 2020, 60, 1351–1356. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Carne, A.; McConnell, M.A. Production of bioactive peptide hydrolysates from deer, sheep and pig plasma using plant and fungal protease preparations. Food Chem. 2015, 176, 54–63. [Google Scholar]
- Yu, P.L.; Vander Linden, D.S.; Sugiarto, H.; Anderson, R.C. Antimicrobial peptides isolated from the blood of farm animals. Anim. Prod. Sci. 2010, 50, 660–669. [Google Scholar] [CrossRef]
- Cui, J.W.; Shi, C.; Xia, P.B.; Ning, K.; Xiang, H.Y.; Xie, Q.H. Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice. Nutrients 2021, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.F.; Zhang, Z.X.; Pei, D.; Wei, J.T.; Di, D.L. The enzyme hydrolysis of Cervus elaphus Linnaeus blood protein and separation & purification of polypeptides. Food Sci. Technol. 2013, 38, 249–252. [Google Scholar]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and assessment of a rat model of fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B.D. In Vitro Antioxidant Activity and In Vivo Anti-Fatigue Effect of Sea Horse (Hippocampus) Peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Cai, X.X.; Wang, J.B.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer Mice. Nutr. 2016, 8, 807. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Shen, C.H.; Huang, Y.Y.; Zhang, X.Q.; Xiao, M.T. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J. Sci. Food Agric. 2017, 97, 4548–4556. [Google Scholar] [CrossRef]
- Wang, P.X.; Zeng, H.L.; Lin, S.L.; Zhang, Z.G.; Zhang, Y.; Hu, J.M. Anti-fatigue activities of hairtail (Trichiurus lepturus) hydrolysate in an endurance swimming mice model. J. Funct. Foods 2020, 74, 104207. [Google Scholar] [CrossRef]
- Xu, M.H.; Liang, R.; Li, Y.; Wang, J.B. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.P.; Zhang, Y.; Liu, Z.; Chen, J.Y.; Zhang, S.Y.; Yang, X.D.; Zhou, H.L. Acute toxicity and anti-fatigue activity of polysaccharide-rich extract from corn silk. Biomed. Pharmacother. 2017, 90, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, F.B.; Zhong, Y.; Li, M.Q.; Chang, Q.; Liao, Y.H.; Liu, X.M.; Pan, R.L. Antioxidant and Anti-Fatigue Constituents of Okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, Y.; Yamaguchi, M.; Noma, T.; Okaya, E.; Itoh, H. Combined effect of arginine, valine, and serine on exercise-induced fatigue in healthy volunteers: A randomized, double-blinded, placebo-controlled crossover study. Nutrients 2019, 11, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Gelly, K.; Jackman, M.L.; Patel, A.; Rennie, M.J. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J. Appl. Physiol. 1999, 86, 1770–1777. [Google Scholar] [CrossRef]
- Varnier, M.; Leese, G.P.; Thompson, J.; Rennie, M.J. Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Am. J. Physiol. 1995, 269, 309–315. [Google Scholar] [CrossRef]
- Audrey, C.; Raquel, R.; Andrea, B.; Thaís, H.; Allan, G.; Jéssica, P.; Amanda, G.; Rafael, L.; Marcelo, R.; Julio, T. Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training. Nutrients 2018, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Raizel, R.; Leite, J.S.M.; Hypólito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Rogero, M.M.; Tirapegui, J.; Pedrosa, R.G.; de Castro, I.A.; Pires, I.S.D.O. Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 2006, 22, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Koo, G.H.; Woo, J.; Kang, S.; Shin, K.O. Effects of Supplementation with BCAA and L-glutamine on Blood Fatigue Factors and Cytokines in Juvenile Athletes Submitted to Maximal Intensity Rowing Performance. J. Phys. Ther. Sci. 2014, 26, 1241–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Risso, E.M.; Amaya-Farfan, J. Bioactivity of food peptides: Biological response of rats to bovine milk whey peptides following acute exercise. Food Nutr. Res. 2017, 61, 1290740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, Y.; Iwasawa, K.; Yamaguchi, M. Acute supplementation of valine reduces fatigue during swimming exercise in rats. Biosci. Biotechnol. Biochem. 2018, 82, 856–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostrzewa, T.; Sahu, K.K.; Gorska-Ponikowska, M.; Tuszynski, J.A.; Kuban-Jankowska, A. Synthesis of small peptide compounds, molecular docking, and inhibitory activity evaluation against phosphatases PTP1B and SHP2. Drug Des. Dev. Ther. 2018, 12, 4139–4147. [Google Scholar] [CrossRef] [Green Version]
- Nishitani, S.; Matsumura, T.; Fujitani, S.; Sonaka, I.; Miura, Y.; Yagasaki, K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem. Biophys. Res. Commun. 2002, 299, 693–696. [Google Scholar] [CrossRef]
- Morifuji, M.; Koga, J.; Kawanaka, K.; Higuchi, M. Branched-Chain amino acid-containing dipeptides, identified from whey protein hydrolysates, stimulate glucose uptake rate in L6 myotubes and isolated skeletal muscles. J. Nutr. Sci. Vitaminol. 2009, 55, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morato, P.N.; Lollo, P.C.B.; Moura, C.S.; Batista, T.M.; Carneiro, E.M.; Amaya-Farfan, J. A dipeptide and an amino acid present in whey protein hydrolysate increase translocation of GLUT-4 to the plasma membrane in Wistar rats. Food Chem. 2013, 139, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushige, T.; Kanegawa, N.; Yamada, A.; Ota, A.; Kanamoto, R.; Ohinata, K. Aromatic amino acid-leucine dipeptides exhibit anxiolytic-like activity in young mice. Neurosci. Lett. 2013, 543, 126–129. [Google Scholar] [CrossRef]
- Kagawa, K.; Matsutaka, H.; Fukuhama, C.; Watanabe, Y.; Fujino, H. Globin digest, acidic protease hydrolysate, inhibits dietary hypertriglyceridemia and Val-Val-Tyr-Pro, one of its constituents, possesses most superior effect. Life Sci. 1996, 58, 1745–1755. [Google Scholar] [CrossRef]
- Du, X.Q.; Xia, Y.; Zhang, X.Q.; Zhang, W.C.; Liang, Z.J.; Lv, J.J.; Wu, Z.B. Optimization of enzymatic hydrolysis process of dried deer blood (Cervus nippon) and evaluation of its anti-exercise fatigue activity. J. Anhui Agric. Univ. 2021, 48, 518–522. [Google Scholar]





| Groups | ST a | BUN b | HG c | MLA d |
|---|---|---|---|---|
| Fr1 | + f | − | + | − |
| Fr2 | + | − | + | − |
| Fr3 | − g | + | + | − |
| Fr4 | − | + | + | + |
| DB hydrolysates | + | + | + | + |
| DB e | + | + | − | + |
| No. | Fr1 | Fr2 | Fr3 | Fr4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT a | Peptides | Area% b | RT | Peptides | Area% | RT | Peptides | Area% | RT | Peptides | Area% | |
| 1 | 2.7 | VQ | 19.0 | 9.9 | VVSA | 9.1 | 11.1 | V L(I)S | 2.7 | 18.0 | L(I)L(I) | 11.6 |
| 2 | 2.9 | AQ | 10.6 | 11.1 | V L(I)S | 11.1 | 13.5 | V L(I) | 9.1 | 19.5 | DQ | 7.4 |
| 3 | 3.3 | L(I)H VN GVH | 5.0 | 12.0 | VNQ(K) | 3.0 | 14.1 | L(I)VT | 3.0 | 20.9 | ARVT | 1.2 |
| 4 | 3.7 | VT | 2.9 | 13.5 | V L(I) | 11.6 | 14.6 | L(I)VE | 2.4 | 21.4 | AYPTT | 4.3 |
| 5 | 3.8 | VAN | 4.4 | 13.8 | FD | 10.1 | 16.5 | L(I)SAL(I) | 1.7 | 22.8 | VVYP FL | 15.2 |
| 6 | 4.2 | TVA L(I)Q | 3.2 | 14.1 | L(I)VT | 5.8 | 17.3 | AF | 5.7 | 24.2 | WT | 4.3 |
| 7 | 4.4 | L(I) | 3.5 | 14.6 | L(I)VE | 3.6 | 18.0 | L(I)L(I) | 26.2 | 25.0 | EVAF | 0.9 |
| 8 | 4.9 | L(I)T | 2.3 | 16.5 | L(I)SAL(I) | 4.8 | 19.5 | DQ | 13.2 | 25.8 | MTH | 2.6 |
| 9 | 8.9 | AL(I) | 4.4 | 17.3 | AF | 5.0 | 27.2 | VNDAF | 3.3 | |||
| 10 | 11.5 | L(I)SE | 2.8 | 18.0 | L(I)L(I) | 3.8 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.-J.; Liu, Y.; Zeng, X.-Y.; Yu, J.; Li, Y.; Du, X.-Q.; Wu, Z.-B.; Hao, S.-L.; Wang, B.-C. Anti-Fatigue Peptides from the Enzymatic Hydrolysates of Cervus elaphus Blood. Molecules 2021, 26, 7614. https://doi.org/10.3390/molecules26247614
Lv J-J, Liu Y, Zeng X-Y, Yu J, Li Y, Du X-Q, Wu Z-B, Hao S-L, Wang B-C. Anti-Fatigue Peptides from the Enzymatic Hydrolysates of Cervus elaphus Blood. Molecules. 2021; 26(24):7614. https://doi.org/10.3390/molecules26247614
Chicago/Turabian StyleLv, Jun-Jiang, Yan Liu, Xiao-Yan Zeng, Jia Yu, Yan Li, Xiao-Qin Du, Zhong-Bao Wu, Shi-Lei Hao, and Bo-Chu Wang. 2021. "Anti-Fatigue Peptides from the Enzymatic Hydrolysates of Cervus elaphus Blood" Molecules 26, no. 24: 7614. https://doi.org/10.3390/molecules26247614
APA StyleLv, J. -J., Liu, Y., Zeng, X. -Y., Yu, J., Li, Y., Du, X. -Q., Wu, Z. -B., Hao, S. -L., & Wang, B. -C. (2021). Anti-Fatigue Peptides from the Enzymatic Hydrolysates of Cervus elaphus Blood. Molecules, 26(24), 7614. https://doi.org/10.3390/molecules26247614