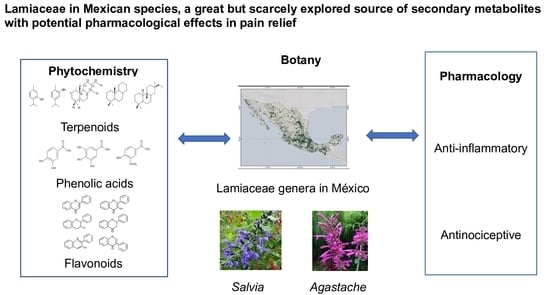
Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief
Abstract
:1. Introduction
2. Results
2.1. Lamiaceae Description
2.2. Geographical Distribution
2.3. Lamiaceae and Some of Its Genera in Mexico
2.4. Pain and Some of Mexican Lamiaceae Genera to Alleviate It
2.5. Salvia Species Used in Pain Relief
2.6. Agastache Species to Alleviate Pain and Inflammation
2.7. Secondary Metabolites Identified in Lamiaceae Species with Analgesic and/or Anti-Inflammatory Activities
2.7.1. Terpenes
Volatile Terpenes
Non-Volatile Terpenes
2.7.2. Phenolic Compounds
Phenolic Acids
Flavonoids
3. Materials and Methods
Literature Survey Databases
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. Stud. Nat. Prod. Chem. 2019, 62, 125–178. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R. Labiatae. In Flowering Plants · Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar] [CrossRef]
- Falcão, D.Q.; Fernandes, S.B.O.; Menezes, F.S. Triterpenos de Hyptis fasciculata Benth. Rev. Bras. Farmacogn. 2003, 13, 81–83. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem. 2008, 1, 751–756. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Astyrakaki, E.; Papaioannou, A.; Askitopoulou, H. References to anesthesia, pain, and analgesia in the Hippocratic collection. Anesth. Analg. 2010, 110, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Orr, P.M.; Shank, B.C.; Black, A.C. The role of pain classification systems in pain management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418. [Google Scholar] [CrossRef]
- Ellison, D.L. Physiology of pain. Crit. Care Nurs. Clin. N. Am. 2017, 29, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J. Overview of pain: Classification and concepts. In Weiner’s Pain Management: A Practical Guide for Clinician; Boswell, M.V., Cole, B.E., Eds.; American Academy of Pain Management: Orlando, FL, USA, 2006; pp. 35–48. [Google Scholar]
- Armstrong, S.A.; Herr, M.J. Physiology Nociception; StatPearls, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK551562/ (accessed on 20 November 2021).
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Rácz, I.; Zimmer, A. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 221–235. [Google Scholar] [CrossRef]
- Dray, A.; Perkins, M. Kinins and Pain. In Handbook of Immunopharmacology; Farmer, S.G., Ed.; Academic Press: London, UK, 1997; pp. 157–172. [Google Scholar] [CrossRef]
- Geppetti, P.; Nassini, R.; Materazzi, S.; Benemei, S. The concept of neurogenic inflammation. BJU Int. 2008, 101, 2–6. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Könner, A.C.; Brüning, J.C. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in Toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Silverman, N.; Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.Y.; Zhu, X.J.; Zhang, Y.N.; Zhou, F.F.; Yang, X.F. Protective effects of apigenin on LPS-induced endometritis via activating Nrf2 signaling pathway. Microb. Pathog. 2018, 123, 139–143. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgis, F.M.S.; Ziaziaris, W.; Munoz, L. Mitogen-activated protein kinase-activated protein kinase 2 in neuroinflammation, heat shock protein 27 phosphorylation, and cell cycle: Role and targeting. Mol. Pharmacol. 2014, 85, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Kokkini, S.; Karousou, R.; Hanlidou, E. HERBS|Herbs of the Labiatae. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Thessaloniki, Greece, 2003; pp. 3082–3090. [Google Scholar] [CrossRef]
- Martínez-Gordillo, M.; Fragoso-Martínez, I.; García-Peña, M.d.R.; Montiel, O. Géneros de Lamiaceae de México, diversidad y endemismo. Rev. Mex. Biodivers. 2013, 84, 30–86. [Google Scholar] [CrossRef] [Green Version]
- Bridi, H.; de Carvalho Meirelles, G.; Lino von Poser, G. Subtribe Hyptidinae (Lamiaceae): A promising source of bioactive metabolites. J. Ethnopharmacol. 2021, 264, 113225. [Google Scholar] [CrossRef]
- Hedge, I.C. A Global survey of the biogeography of the Labiatae. In Advances in Labiate Science; Harley, R.M., Reynolds, T., Eds.; Royal Botani Gardens, Kew: Richmond, UK, 1992; Volume 6, pp. 7–17. [Google Scholar]
- Li, B.; Olmstead, R.G. Two new subfamilies in Lamiaceae. Phytotaxa 2017, 313, 222–226. [Google Scholar] [CrossRef]
- Callmander, M.W.; Phillipson, P.B. New combinations and typifications in Vitex (Lamiaceae) from Madagascar. Candollea 2018, 73, 131–136. [Google Scholar] [CrossRef]
- Xi-wen, L.; Hedge, I.C. Lamiaceae Chun Xing Ke. Flora China 1994, 17, 50–299. [Google Scholar]
- Valverde, R.M. Labiates (Lamiaceae) of Chile. An. Jard. Bot. Madr. 2018, 75, e067. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gordillo, M.; Bedolla-García, B.; Cornejotenorio, G.; Fragoso-Martínez, I.; García-Peña, M.D.R.; González-Gallegos, J.G.; Lara-Cabrera, S.I.; Zamudio, S. Lamiaceae of Mexico. Bot. Sci. 2017, 95, 780–806. [Google Scholar] [CrossRef] [Green Version]
- González-Gallegos, J.G.; Bedolla-García, B.Y.; Cornejo-Tenorio, G.; Fernández-Alonso, J.L.; Fragoso-Martínez, I.; García-Peña, M.D.R.; Harley, R.M.; Klitgaard, B.; Martínez-Gordillo, M.J.; Wood, J.R.I.; et al. Richness and distribution of Salvia subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 2020, 181, 831–856. [Google Scholar] [CrossRef]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.C.; Xiang, C.L.; Ma, Z.H.; Tan, Y.H.; Zhang, D.X. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef] [Green Version]
- Hanlidou, E.; Karousou, R.; Kleftoyanni, V.; Kokkini, S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 2004, 91, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Kleftoyanni, V.; Kokkini, S. The Labiatae plants used traditionally in Thessaloniki. Bocconea 2003, 16, 1117–1121. [Google Scholar]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Gharedaghi, M.; Hadjiakhoondi, A.; Sharifzadeh, M.; Khanavi, M. Antinociceptive effect of extracts of Marrubium astracanicum Jacq. aerial parts. Avicenna J. Phytomed. 2017, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- De Lima, A.C.B.; Paixão, M.S.; Melo, M.; Santana, M.T.D.; Damascena, N.P.; Dias, A.S.; Porto, Y.C.B.S.; Fernandes, X.A.; Santos, C.C.S.; Lima, C.A.; et al. Orofacial antinociceptive effect and antioxidant properties of the hydroethanol extract of Hyptis fruticosa salmz ex Benth. J. Ethnopharmacol. 2013, 146, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Anjos, K.S.; Araújo-Filho, H.G.; Duarte, M.C.; Costa, V.C.O.; Tavares, J.F.; Silva, M.S.; Almeida, J.R.G.S.; Souza, N.A.C.; Rolim, L.A.; Menezes, I.R.A.; et al. HPLC-DAD analysis, antinociceptive and anti-inflammatory properties of the ethanolic extract of Hyptis umbrosa in mice. EXCLI J. 2017, 14, 14–24. [Google Scholar] [CrossRef]
- Arzi, A.; Namjouyan, F.; Sarahoodi, S.; Khorasgani, Z.N.; Macvandi, E. The study of antinociceptive effect of hydroalcoholic extract of Teucrium oliverianum (A plant use in Southern Iranian traditional medicine) in rat by formalin test. Pak. J. Biol. Sci. 2011, 14, 1066–1069. [Google Scholar] [CrossRef]
- Miri, A.; Sharifi-Rad, J.; Tabrizian, K.; Nasiri, A.A. Antinociceptive and anti-inflammatory activities of Teucrium persicum Boiss. extract in mice. Scientifica 2015, 2015, 972827. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.M.M.; Shah, S.M.H. Phytochemicals, antioxidant, antinociceptive and anti-inflammatory potential of the aqueous extract of Teucrium stocksianum bioss. BMC Complement. Altern. Med. 2015, 15, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Qu, F.; Zhang, H.J.; Zhuge, X.H.; Cheng, L.Z. Comparison of anti-inflammatory and anti-nociceptive activities of Curcuma wenyujin Y.H. Chen et C. Ling and Scutellaria baicalensis Georgi. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaee-Asl, M.; Sabour, M.; Nikoui, V.; Ostadhadi, S.; Bakhtiarian, A. The study of analgesic effects of Leonurus cardiaca L. in mice by formalin, tail flick and hot plate tests. Int. Sch. Res. Not. 2014, 2014, 687697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirova, K.M.; Dimitrova, P.; Marchev, A.S.; Aneva, I.Y.; Georgiev, M.I. Clinopodium vulgare L. (wild basil) extract and its active constituents modulate cyclooxygenase-2 expression in neutrophils. Food Chem. Toxicol. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant effects of Clinopodium mexicanum Benth. Govaerts (Lamiaceae) on the central nervous system. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef]
- Bajalan, I.; Zand, M.; Goodarzi, M.; Darabi, M. Antioxidant activity and total phenolic and flavonoid content of the extract and chemical composition of the essential oil of Eremostachys laciniata collected from Zagros. Asian Pac. J. Trop. Biomed. 2017, 7, 144–146. [Google Scholar] [CrossRef]
- Zhu, Q.-F.; Wang, Y.-Y.; Jiang, W.; Qu, H.-B. Three new norlignans from Glechoma longituba. J. Asian Nat. Prod. Res. 2013, 15, 258–264. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Rivas-Morales, C.; Carranza-Rosales, P.; Mendoza, S.; Schmeda-Hirschmann, G. Free Radical Scavengers from the Mexican Herbal Tea “Poleo” (Hedeoma drummondii). Z. Naturforsch. Sect. C J. Biosci. 2008, 63, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadé, M.M.; Schinella, G.R.; Fioravanti, D.E.; Alfiotournier, H. Antioxidant and cytotoxic properties of an aqueous extract from the Argentinean plant Hedeoma multiflorum. Pharm. Biol. 2011, 49, 633–639. [Google Scholar] [CrossRef]
- Pal, M.; Singh, M.; Chaudhuri, P.K.; Sharma, R.P.; Singh, N. Antiinflammatory activity of Holmskioldia sanguinea extracts. Phyther. Res. 1996, 10, 357–358. [Google Scholar] [CrossRef]
- Begum, A.; Sama, V.; Dodle, J.P. Study of antinociceptive effects on acute pain treated by bioactive fractions of Hyptis suaveolens. J. Acute Dis. 2016, 5, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Simões, R.R.; Coelho, I.d.S.; Junqueira, S.C.; Pigatto, G.R.; Salvador, M.J.; Santos, A.R.S.; de Faria, F.M. Oral treatment with essential oil of Hyptis spicigera Lam. (Lamiaceae) reduces acute pain and inflammation in mice: Potential interactions with transient receptor potential (TRP) ion channels. J. Ethnopharmacol. 2017, 200, 8–15. [Google Scholar] [CrossRef]
- Trouillas, P.; Calliste, C.A.; Allais, D.P.; Simon, A.; Marfak, A.; Delage, C.; Duroux, J.L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. “Budrovka”: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtyniak, K.; Szymański, M.; Matławska, I. Leonurus cardiaca L. (Motherwort): A review of its phytochemistry and pharmacology. Phyther. Res. 2013, 27, 1115–1120. [Google Scholar] [CrossRef]
- El-Ansari, M.A.; Aboutabl, E.A.; Farrag, A.R.H.; Sharaf, M.; Hawas, U.W.; Soliman, G.M.; El-Seed, G.S. Phytochemical and pharmacological studies on Leonotis leonurus. Pharm. Biol. 2009, 47, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Sadhu, S.K.; Hasan, C.M. Preliminary antinociceptive, antioxidant and cytotoxic activities of Leucas aspera root. Fitoterapia 2007, 78, 552–555. [Google Scholar] [CrossRef]
- Meyre-Silva, C.; Cechinel-Filho, V. A Review of the Chemical and Pharmacological Aspects of the Genus Marrubium. Curr. Pharm. Des. 2010, 16, 3503–3518. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e0114767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, L.; Bello, R.; Primo-Yúfera, E.; Esplugues, J. Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phyther. Res. 2002, 16, 10–13. [Google Scholar] [CrossRef]
- Trakoontivakorn, G.; Tangkanakul, P.; Nakahara, K. Changes of antioxidant capacity and phenolics in Ocimum herbs after various cooking methods. Jpn. Agric. Res. Q. 2012, 46, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Bae, A.H.; Kim, G.; Seol, G.H.; Lee, S.B.; Lee, J.M.; Chang, W.; Min, S.S. Delta- and mu-opioid pathways are involved in the analgesic effect of Ocimum basilicum L in mice. J. Ethnopharmacol. 2020, 250, 112471. [Google Scholar] [CrossRef] [PubMed]
- Algieri, F.; Zorrilla, P.; Rodriguez-Nogales, A.; Garrido-Mesa, N.; Bañuelos, Ó.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Zarzuelo, A.; Utrilla, M.P.; et al. Intestinal anti-inflammatory activity of hydroalcoholic extracts of Phlomis purpurea L. and Phlomis lychnitis L. in the trinitrobenzenesulphonic acid model of rat colitis. J. Ethnopharmacol. 2013, 146, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Uren, M.C.; Tepe, B.; Cengiz, M.; Kocak, M.S. Phenolic content, enzyme inhibitory and antioxidative activity potentials of Phlomis nissolii and P. pungens var. pungens. Ind. Crop. Prod. 2014, 62, 333–340. [Google Scholar] [CrossRef]
- Narayanan, N.; Thirugnanasambantham, P.; Viswanathan, S.; Reddy, M.K.; Vijayasekaran, V.; Sukumar, E. Antipyretic, antinociceptive and anti-inflammatory activity of Premna herbacea roots. Fitoterapia 2000, 71, 147–153. [Google Scholar] [CrossRef]
- Azad, R.; Babu, N.K.; Gupta, A.D.; Reddanna, P. Evaluation of anti-inflammatory and immunomodulatory effects of Premna integrifolia extracts and assay-guided isolation of a COX-2/5-LOX dual inhibitor. Fitoterapia 2018, 131, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; Wurglics, M.; Schubert-Zsilavecz, M. Prunella vulgaris L. active components and their hypoglycemic and antinociceptive effects in alloxan-induced diabetic mice. Biomed. Pharmacother. 2016, 84, 1008–1018. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Martínez, A.L.; González-Trujano, M.E.; Chávez, M.; Pellicer, F. Antinociceptive effectiveness of triterpenes from rosemary in visceral nociception. J. Ethnopharmacol. 2012, 142, 28–34. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phyther. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.R.A.; Kanazawa, L.K.S.; Neves, T.L.M.H.D.; Silva, C.F.D.; Horst, H.; Pizzolatti, M.G.; Santos, A.R.S.; Baggio, C.H.; Werner, M.F.D.P. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef]
- Fan, M.; Luo, D.; Peng, L.Y.; Li, X.N.; Wu, X.D.; Ji, X.; Zhao, Q.S. Neo-clerodane diterpenoids from aerial parts of Salvia hispanica L. and their cardioprotective effects. Phytochemistry 2019, 166, 112065. [Google Scholar] [CrossRef] [PubMed]
- Cuong, T.D.; Hung, T.M.; Lee, J.S.; Weon, K.Y.; Woo, M.H.; Min, B.S. Anti-inflammatory activity of phenolic compounds from the whole plant of Scutellaria indica. Bioorg. Med. Chem. Lett. 2015, 25, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Central nervous system diseases and Scutellaria: A review of current mechanism studies. Biomed. Pharmacother. 2018, 102, 185–195. [Google Scholar] [CrossRef]
- Cavalcanti, M.R.M.; Passos, F.R.S.; Monteiro, B.S.; Gandhi, S.R.; Heimfarth, L.; Lima, B.S.; Nascimento, Y.M.; Duarte, M.C.; Araujo, A.A.S.; Menezes, I.R.A.; et al. HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement. J. Ethnopharmacol. 2021, 265, 113338. [Google Scholar] [CrossRef]
- Bardakci, H.; Cevik, D.; Barak, T.H.; Gozet, T.; Kan, Y.; Kirmizibekmez, H. Secondary metabolites, phytochemical characterization and antioxidant activities of different extracts of Sideritis congesta P.H. Davis et Hub.-Mor. Biochem. Syst. Ecol. 2020, 92, 104120. [Google Scholar] [CrossRef]
- Khanavi, M.; Sharifzadeh, M.; Hadjiakhoondi, A.; Shafiee, A. Phytochemical investigation and anti-inflammatory activity of aerial parts of Stachys byzanthina C. Koch. J. Ethnopharmacol. 2005, 97, 463–468. [Google Scholar] [CrossRef]
- Maleki, N.; Garjani, A.; Nazemiyeh, H.; Nilfouroushan, N.; Eftekhar Sadat, A.T.; Allameh, Z.; Hasannia, N. Potent anti-inflammatory activities of hydroalcoholic extract from aerial parts of Stachys inflata on rats. J. Ethnopharmacol. 2001, 75, 213–218. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Reddy, V.P.; Vital, K.R.; Varsha, P.; Satyam, S. Review on Thymus vulgaris traditional uses and pharmacological properties. Med. Aromat. Plants 2014, 3, 167. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hamann, F.R.; Zago, A.M.; Rossato, M.F.; Beck, V.R.; Mello, C.F.; de Brum, T.F.; de Carvalho, L.M.; Faccin, H.; Oliveira, S.M.; Rubin, M.A. Antinociceptive and antidepressant-like effects of the crude extract of Vitex megapotamica in rats. J. Ethnopharmacol. 2016, 192, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Jurca, T.; Baldea, I.; Filip, G.A.; Olteanu, D.; Clichici, S.; Pallag, A.; Vicaş, L.; Marian, E.; Micle, O.; Crivii, C.B.; et al. A phytocomplex consisting of Tropaeolum majus L. and Salvia officinalis L. extracts alleviate the inflammatory response of dermal fibroblasts to bacterial lipopolysaccharides. Oxid. Med. Cell. Longev. 2020, 2020, 8516153. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Trujano, M.E.; Pena, E.I.; Martinez, A.L.; Moreno, J.; Guevara-Fefer, P.; Deciga-Campos, M.; Lopez-Munoz, F.J. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Martinez, A.L.; Gonzalez-Trujano, M.E.; Chavez, M.; Pellicer, F.; Moreno, J.; Lopez-Munoz, F.J. Hesperidin produces antinociceptive response and synergistic interaction with ketorolac in an arthritic gout-type pain in rats. Pharmacol. Biochem. Behav. 2011, 97, 683–689. [Google Scholar] [CrossRef]
- Ventura-Martínez, R.; Rivero-Osorno, O.; Gómez, C.; González-Trujano, M.E. Spasmolytic activity of Rosmarinus officinalis L. involves calcium channels in the guinea pig ileum. J. Ethnopharmacol. 2011, 137, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Villalobos, K.L.; Déciga-Campos, M.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Martínez-Salazar, M.F.; Ramírez-Cisneros, M.d.l.Á.; Rios, M.Y.; López-Muñoz, F.J. Synergistic antinociceptive interaction of Syzygium aromaticum or Rosmarinus officinalis coadministered with ketorolac in rats. Biomed. Pharmacother. 2017, 94, 858–864. [Google Scholar] [CrossRef]
- Cunningham, C.W.; Rothman, R.B.; Prisinzano, T.E. Neuropharmacology of the naturally occurring κ-opioid hallucinogen salvinorin A. Pharmacol. Rev. 2011, 63, 316–347. [Google Scholar] [CrossRef] [Green Version]
- Tlacomulco-Flores, L.L.; Déciga-Campos, M.; González-Trujano, M.E.; Carballo-Villalobos, A.I.; Pellicer, F. Antinociceptive effects of Salvia divinorum and bioactive salvinorins in experimental pain models in mice. J. Ethnopharmacol. 2020, 248, 112276. [Google Scholar] [CrossRef] [PubMed]
- Simón-Arceo, K.; González-Trujano, M.E.; Coffeen, U.; Fernández-Mas, R.; Mercado, F.; Almanza, A.; Contreras, B.; Jaimes, O.; Pellicer, F. Neuropathic and inflammatory antinociceptive effects and electrocortical changes produced by Salvia divinorum in rats. J. Ethnopharmacol. 2017, 206, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Aviello, G.; Palazzo, E.; De Chiaro, M.; Gatta, L.; Boccella, S.; Marabese, I.; Zjawiony, J.K.; Capasso, R.; et al. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol. Pain 2012, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Coffeen, U.; Canseco-Alba, A.; Simón-Arceo, K.; Almanza, A.; Mercado, F.; León-Olea, M.; Pellicer, F. Salvinorin A reduces neuropathic nociception in the insular cortex of the rat. Eur. J. Pain 2018, 22, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, G.F.; González-Trujano, M.E.; Martínez-Gordillo, M.J.; Miguel-Chávez, R.S.; Basurto-Peña, F.A.; Dorazco-González, A.; Aguirre-Hernández, E. Amarisolide A and pedalitin as bioactive compounds in the antinociceptive effects of Salvia circinata (Lamiaceae). Bot. Sci. 2019, 97, 355–365. [Google Scholar] [CrossRef]
- Moreno-Pérez, F.; Hernandez-Leon, A.; Valle Dorado, M.G.; Cano Martínez, A.; Narváez-González, F.; Aguirre Hernández, E.; Salgado-Ceballos, H.; González-Trujano, M.E. Neo-clerodane diterpenic influence in the antinociceptive and anti-inflammatory properties of Salvia circinnata Cav. J. Ethnopharmacol. 2021, 268, 113550. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; González-Andrade, M.; Bye, R.; Linares, E.; Mata, R. α-glucosidase inhibitors from Salvia circinata. J. Nat. Prod. 2017, 80, 1584–1593. [Google Scholar] [CrossRef]
- Salinas-Arellano, E.; Pérez-Vásquez, A.; Rivero-Cruz, I.; Torres-Colin, R.; González-Andrade, M.; Rangel-Grimaldo, M.; Mata, R. Flavonoids and terpenoids with PTP-1B inhibitory properties from the infusion of Salvia amarissima ortega. Molecules 2020, 25, 3530. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Leon, A.; Fernández-Guasti, A.; González-Trujano, M.E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats. Eur. J. Pain 2016, 20, 274–283. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; González-Trujano, M.E.; Fernández-Guasti, A. The anxiolytic-like effect of rutin in rats involves GABAA receptors in the basolateral amygdala. Behav. Pharmacol. 2017, 28, 303–312. [Google Scholar] [CrossRef]
- Bautista, E.; Fragoso-Serrano, M.; Ortiz-Pastrana, N.; Toscano, R.A.; Ortega, A. Structural elucidation and evaluation of multidrug-resistance modulatory capability of amarissinins A–C, diterpenes derived from Salvia amarissima. Fitoterapia 2016, 114, 1–6. [Google Scholar] [CrossRef]
- Ortiz-Mendoza, N.; Zavala-Ocampo, L.M.; Martínez-Gordillo, M.J.; González-Trujano, M.E.; Peña, F.A.B.; Bazany-Rodríguez, I.J.; Chávez, J.A.R.; Dorazco-González, A.; Aguirre-Hernández, E. Antinociceptive and anxiolytic-like effects of a neo-clerodane diterpene from Salvia semiatrata aerial parts. Pharm. Biol. 2020, 58, 620–629. [Google Scholar] [CrossRef]
- Weisenberg, M. Cognitive aspects of pain. In Wall & Melzack’s Textbook of Pain; Wall, P.D., Melzack, R., Eds.; Churchill-Livingstone: London, UK, 1984; p. 1588. [Google Scholar]
- Estrada-Reyes, R.; López-Rubalcava, C.; Ferreyra-Cruz, O.A.; Dorantes-Barrón, A.M.; Heinze, G.; Moreno Aguilar, J.; Martínez-Vázquez, M. Central nervous system effects and chemical composition of two subspecies of Agastache mexicana; An ethnomedicine of Mexico. J. Ethnopharmacol. 2014, 153, 98–110. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Ventura-Martínez, R.; Chávez, M.; Díaz-Reval, I.; Pellicer, F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 2012, 78, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Martínez, R.; Rodríguez, R.; González-Trujano, M.E.; Ángeles-López, G.E.; Déciga-Campos, M.; Gómez, C. Spasmogenic and spasmolytic activities of Agastache mexicana ssp. mexicana and A. mexicana ssp. xolocotziana methanolic extracts on the guinea pig ileum. J. Ethnopharmacol. 2017, 196, 58–65. [Google Scholar] [CrossRef]
- González-Ramírez, A.E.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J. Anti-nociceptive and anti-inflammatory activities of the Agastache mexicana extracts by using several experimental models in rodents. J. Ethnopharmacol. 2012, 142, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Verano, J.; González-Trujano, M.E.; Déciga-Campos, M.; Ventura-Martínez, R.; Pellicer, F. Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol. Biochem. Behav. 2013, 110, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products: Drug Discovery and Therapeutic Medicine; Zhang, L., Demian, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 197–227. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Shanoo, S.; Rengasamy Kannan, R.R.; Fawzi, M.M. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Antiinflammatory potential of medicinal plants: A source for therapeutic secondary metabolites. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2018; pp. 131–183. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- De Souza Siqueira, Q.J.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.D.S.; Quintans, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes modulating cytokines—A review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef]
- Ghosh, M.; Schepetkin, I.A.; Özek, G.; Özek, T.; Khlebnikov, A.I.; Damron, D.S.; Quinn, M.T. Essential oils from Monarda fistulosa: Chemical composition and activation of transient receptor potential A1 (TRPA1) channels. Molecules 2020, 25, 4873. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.M.; Ullah, F.; Shah, S.M.H.; Zahoor, M.; Sadiq, A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the North West of Pakistan. BMC Complement. Altern. Med. 2012, 12, 244. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, M.; Souza, E.P.; Soares, P.M.G.; Miranda, A.V.; Matos, F.J.A.; Criddle, D.N. Antinociceptive properties of the essential oil of Ocimum gratissimum L. (Labiatae) in mice. Braz. J. Med. Biol. Res. 2003, 36, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-pinene exhibits anti-Inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of anti-inflammatory activity of Citrus latifolia Tanaka essential oil and limonene in experimental mouse models. Evid.-Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.-J.; Lee, N.H.; Hyun, C.-G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Batista, P.A.; De Paula Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; Da Silva Brum, L.F.; Story, G.M.; Santos, A.R.S. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Anuradha, C.V. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Chessa, M.L.; Moretti, M.D.L.; Serra, G.; Pippia, P. (-)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Rao, V.S.N.; Menezes, A.M.S.; Viana, G.S.B. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990, 42, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- De Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; Silva, J.C.; Bonjardim, L.R.; Lucca Júnior, W.; de Ferro, J.N.d.S.; Barreto, E.d.O.; Santos, F.E.; dos Soares, M.B.P.; et al. The anti-hyperalgesic and anti-inflammatory profiles of p -cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 2015, 53, 1583–1590. [Google Scholar] [CrossRef]
- Santos, W.B.R.; Melo, M.A.O.; Alves, R.S.; de Brito, R.G.; Rabelo, T.K.; Prado, L.d.S.; Silva, V.K.d.S.; Bezerra, D.P.; de Menezes-Filho, J.E.R.; Souza, D.S.; et al. p-Cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine 2019, 61, 152836. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2002, 19, 571–579. [Google Scholar] [CrossRef]
- Xu, Z.H.; Wang, C.; Fujita, T.; Jiang, C.Y.; Kumamoto, E. Action of thymol on spontaneous excitatory transmission in adult rat spinal substantia gelatinosa neurons. Neurosci. Lett. 2015, 606, 94–99. [Google Scholar] [CrossRef]
- Liu, S.D.; Song, M.H.; Yun, W.; Lee, J.H.; Kim, H.B.; Cho, J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019, 98, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Almeida, J.R.G.S.; Quintans, J.S.S.; Gopalsamy, R.G.; Shanmugam, S.; Serafini, M.R.; Oliveira, M.R.C.; Silva, B.A.F.; Martins, A.O.B.P.B.; Castro, F.F.; et al. Enhancement of orofacial antinociceptive effect of carvacrol, a monoterpene present in oregano and thyme oils, by β-cyclodextrin inclusion complex in mice. Biomed. Pharmacother. 2016, 84, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a natural sesquiterpene, attenuates neuropathic pain and depressive-like behavior in experimental diabetic mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Leon, A.; González-Trujano, M.E.; Narváez-González, F.; Pérez-Ortega, G.; Rivero-Cruz, F.; Aguilar, M.I. Role of β-caryophyllene in the antinociceptive and anti-inflammatory effects of Tagetes lucida Cav. essential oil. Molecules 2020, 25, 675. [Google Scholar] [CrossRef] [Green Version]
- Aldred, E. Terpenes. In Pharmacology; Aldred, E., Buck, C., Kenneth, V., Eds.; Churchill Livingstone: London, UK, 2009; pp. 167–174. [Google Scholar] [CrossRef]
- Vestri Alvarenga, S.; Pierre Gastmans, J.; Do Vale Rodrigues, G.; Moreno, P.R.H.; De Paulo Emerenciano, V. A computer-assisted approach for chemotaxonomic studies—Diterpenes in Lamiaceae. Phytochemistry 2001, 56, 583–595. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef] [Green Version]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as a major bioactive component in Rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, I.T.; Park, H.J.; Kim, H.M.; Choi, J.H.; Lee, K.T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Nam, J.-H.; Jung, H.-J.; Lee, M.-S.; Lee, K.-T.; Jung, M.-H.; Choi, J.-W. Inhibitory effect of euscaphic acid and tormentic acid from the roots of Rosa rugosa on high fat diet-induced obesity in the rat. Korean J. Pharmacogn. 2005, 36, 324–331. [Google Scholar]
- De las Heras, B.; Navarro, A.; Godoy, A.; Villar, A.M. Inhibition of the induction of nitric oxide synthase in J774 macrophages by andalusol, a diterpenoid from Sideritis foetens Clem. Pharm. Pharmacol. Lett. 1997, 7, 111–112. [Google Scholar]
- De Las Heras, B.; Navarro, A.; Díaz-Guerra, M.J.; Bermejo, P.; Castrillo, A.; Boscá, L.; Villar, A. Inhibition of NOS-2 expression in macrophages through the inactivation of NF-κB by andalusol. Br. J. Pharmacol. 1999, 128, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, Y. The therapeutic effect of tanshinone IIA on Propionibacterium acnes -induced inflammation in vitro. Dermatol. Ther. 2018, 31, 12716. [Google Scholar] [CrossRef]
- McCurdy, C.R.; Sufka, K.J.; Smith, G.H.; Warnick, J.E.; Nieto, M.J. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol. Biochem. Behav. 2006, 83, 109–113. [Google Scholar] [CrossRef]
- Pittaluga, A.; Olivero, G.; Di Prisco, S.; Merega, E.; Bisio, A.; Romussi, G.; Grilli, M.; Marchi, M. Effects of the neoclerodane hardwickiic acid on the presynaptic opioid receptors which modulate noradrenaline and dopamine release in mouse central nervous system. NeuroChem. Int. 2013, 62, 354–359. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-silva, C.; Muller, L.; Cani, G.S.; Santos, A.R.S.; Yunes, R. Antinociceptive properties of mixture of α-amyrin and β-amyrin triterpenes: Evidence for participation of Protein Kinase C and Protein Kinase A pathways. J Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Quintão, N.L.M.; Rocha, L.W.; Silva, G.F.; Reichert, S.; Claudino, V.D.; Lucinda-Silva, R.M.; Malheiros, A.; Souza, M.M.; De, Filho, V.C.; Bresolin, T.M.B.; et al. Contribution of α,β-amyrenone to the anti-Inflammatory and antihypersensitivity effects of Aleurites moluccana (L.) Willd. BioMed Res. Int. 2014, 2014, 636839. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, A.; Silveira, P.C.; Nesi, R.T.; Pinho, R.A. β-Amyrin, a pentacyclic triterpene, exhibits anti-fibrotic, anti-inflammatory, and anti-apoptotic effects on dimethyl nitrosamine–induced hepatic fibrosis in male rats. Hum. Exp. Toxicol. 2017, 36, 113–122. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.N.; Han, S.N.; Kim, H.K. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 228–235. [Google Scholar] [CrossRef]
- Lo, A.H.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages. Carcinogenesis 2002, 23, 983–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase. J. Pharmacol. Exp. Ther. 2012, 342, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Subbaramaiah, K.; Cole, P.; Dannenberg, A.J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2002, 62, 2522–2530. [Google Scholar]
- Maia, J.L.; Lima-Júnior, R.C.P.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S.N. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: Possible mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Sim, Y.B.; Kang, Y.J.; Kim, S.S.; Kim, C.H.; Kim, S.J.; Suh, H.W. Mechanisms involved in the antinociceptive effects of orally administered oleanolic acid in the mouse. Arch. Pharm. Res. 2013, 36, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Costa, J.F.; Barbosa-Filho, J.M.; De Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-Dos-Santos, R.; Pontes De Carvalho, L.C.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.C.; Peng, W.H.; Chiu, T.H.; Lai, S.C.; Lee, C.Y. Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am. J. Chin. Med. 2011, 39, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Sedano-Partida, M.D.; dos Santos, K.P.; Sala-Carvalho, W.R.; Silva-Luz, C.L.; Furlan, C.M. A review of the phytochemical profiling and biological activities of Hyptis Jacq.: A Brazilian native genus of Lamiaceae. Braz. J. Bot. 2020, 43, 213–228. [Google Scholar] [CrossRef]
- Shin, K.M.; Kim, I.T.; Park, Y.M.; Ha, J.; Choi, J.W.; Park, H.J.; Lee, Y.S.; Lee, K.T. Anti-inflammatory effect of caffeic acid methyl ester and its mode of action through the inhibition of prostaglandin E2, nitric oxide and tumor necrosis factor-α production. Biochem. Pharmacol. 2004, 68, 2327–2336. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Notarius, C.F.; Chan, C.T.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am. J. Physiol. Hear. Circ. Physiol. 2008, 294, H605–H612. [Google Scholar] [CrossRef] [Green Version]
- Spiteller, G. Lipid peroxidation in aging and age-dependent diseases. Exp. Gerontol. 2001, 36, 1425–1457. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine 2018, 40, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.B.; Wang, Y.; He, C.; Yang, Y.; Wan, J.B. Gallic acid, a natural polyphenol, protects against tert-butyl hydroperoxide- induced hepatotoxicity by activating ERK-Nrf2-Keap1-mediated antioxidative response. Food Chem. Toxicol. 2018, 119, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Muthuraman, A. Ameliorative effect of gallic acid in paclitaxel-induced neuropathic pain in mice. Toxicol. Rep. 2019, 6, 505–513. [Google Scholar] [CrossRef]
- Trevisan, G.; Rossato, M.F.; Tonello, R.; Hoffmeister, C.; Klafke, J.Z.; Rosa, F.; Pinheiro, K.V.; Pinheiro, F.V.; Boligon, A.A.; Athayde, M.L.; et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 679–689. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phyther. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Haranishi, Y.; Kataoka, K.; Takahashi, Y.; Terada, T.; Nakamura, M.; Sata, T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014, 723, 459–464. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, E.M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sim, Y.B.; Choi, S.M.; Seo, Y.J.; Kwon, M.S.; Lee, J.K.; Suh, H.W. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch. Pharm. Res. 2009, 32, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, S.; Li, H.; Jiang, X.; Yin, P.; Wan, C.; Liu, X.; Liu, F.; Xu, J. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J. Dairy Sci. 2014, 97, 2856–2865. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, A.; Shanbhag, R.; Chamallamudi, M.R.; Singh, V.P.; Mudgal, J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur. J. Pharmacol. 2011, 666, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Yrbas, M.D.L.A.; Morucci, F.; Alonso, R.; Gorzalczany, S. Pharmacological mechanism underlying the antinociceptive activity of vanillic acid. Pharmacol. Biochem. Behav. 2015, 132, 88–95. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting inflammatory mediators with ferulic acid, a dietary polyphenol, for the suppression of monosodium urate crystal-induced inflammation in rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef]
- Nile, S.H.; Ko, E.Y.; Kim, D.H.; Keum, Y.S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Braz. Pharmacogn. 2016, 26, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Maggini, R.; Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop Sci. 2013, 7, 321–327. [Google Scholar]
- Salmaki, Y.; Zarre, S.; Govaerts, R.; Bräuchler, C. A taxonomic revision of the genus Stachys (Lamiaceae: Lamioideae) in Iran. Bot. J. Linn. Soc. 2012, 170, 573–617. [Google Scholar] [CrossRef] [Green Version]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Sun, Z.; Zhang, X.; Yuan, J.; Wu, H.; Yang, J.; Xu, X. Clerodendranoic acid, a new phenolic acid from Clerodendranthus spicatus. Molecules 2012, 17, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet. Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Mansourabadi, A.H.; Sadeghi, H.M.; Razavi, N.; Rezvani, E. Anti-inflammatory and analgesic properties of Salvigenin, Salvia officinalis flavonoid extracted. Adv. Herb. Med. 2015, 2, 31–41. [Google Scholar]
- Rao, Y.K.; Fang, S.H.; Hsieh, S.C.; Yeh, T.H.; Tzeng, Y.M. The constituents of Anisomeles indica and their anti-inflammatory activities. J. Ethnopharmacol. 2009, 121, 292–296. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khuwaja, G.; Srivastawa, P.; Khan, M.B.; Raza, S.S.; Javed, H.; Vaibhav, K.; Khan, A.; et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009, 1292, 123–135. [Google Scholar] [CrossRef]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 771, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Douglas, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filho, A.W.; Filho, V.C.; Olinger, L.; De Souza, M.M. Quercetin: Further investigation of its antinociceptive properties and mechanisms of action. Arch. Pharm. Res. 2008, 31, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Shieh, T.M.; Wang, K.L.; Huang, T.C.; Hsia, S.M. Quercetin, a main flavonoid in onion, inhibits the PGF2α-induced uterine contraction in vitro and in vivo. J. Funct. Foods 2015, 19, 495–504. [Google Scholar] [CrossRef]
- Martinez, A.L.; Gonzalez-Trujano, M.E.; Aguirre-Hernandez, E.; Moreno, J.; Soto-Hernandez, M.; Lopez-Munoz, F.J. Antinociceptive activity of Tilia americana var mexicana inflorescences and quercetin in the formalin test and in an arthritic pain model in rats. Neuropharmacology 2009, 56, 564–571. [Google Scholar] [CrossRef]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, S.; Vadnere, G.P.; Patil, K.D.; Patil, T.P. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. J. Tradit. Complement. Med. 2020, 10, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, X.; Xu, J.; Wang, T.; Liang, T.; Xu, X.; Ma, C.; Xu, Z.; Wang, W.; Li, H.; et al. Luteolin alleviates neuroinflammation via downregulating the TLR4/TRAF6/NF-κB pathway after intracerebral hemorrhage. Biomed. Pharmacother. 2020, 126, 110044. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Villalobos, A.I.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J. Antihyperalgesic effect of hesperidin improves with diosmin in experimental neuropathic pain. BioMed Res. Int. 2016, 2016, 8263463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loscalzo, L.M.; Wasowski, C.; Paladini, A.C.; Marder, M. Opioid receptors are involved in the sedative and antinociceptive effects of hesperidin as well as in its potentiation with benzodiazepines. Eur. J. Pharmacol. 2008, 580, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: Possible role of PPAR-γ Bax/Bcl-2, and caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.F.; Sun, W.X. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med. Sci. Monit. 2017, 23, 3746–3751. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Yu, D.H.; Ma, C.H.; Yue, Z.Q.; Yao, X.; Mao, C.M. Protective effect of naringenin against lipopolysaccharide-induced injury in normal human bronchial epithelium via suppression of MAPK signaling. Inflammation 2014, 38, 195–204. [Google Scholar] [CrossRef]



| Scientific Name | Medical Properties | Used Plant Organs | Preparation | ||
|---|---|---|---|---|---|
| Analgesic | Anti- Inflammatory | Antioxidant | |||
| Clinopodium vulgare L. [50] | X | Aerial parts | Hydroalcoholic extract | ||
| C. mexicanun (Benth.) Govaerts [51] | X | Leaves | Organic extracts | ||
| Eremostachys laciniata (L.) Bunge. [52] | X | Aerial parts | Hydrodistillation, Methanol extract | ||
| Glechoma longituba Kupr. [53] | X | Aerial parts | Infusion | ||
| Hedeoma drummondii Benth. [54] | X | Aerial parts | Maceration | ||
| H. multiflorum Benth. [55] | X | Aerial parts | Infusion | ||
| Holmskioldia sanguinea Retz. [56] | X | Leaves | Methanol extract | ||
| Hyptis suaveolens (L.) Poit. [57] | X | Aerial parts | Hydroalcoholic extract | ||
| H. spicigera Lam. [58] | X | X | Aerial parts | Hydrodistillation | |
| Lamiumálbum L. [59] | X | X | Aerial parts | Hydroalcoholic extract | |
| Lavandula angustifolia Mill. [60,61] | X | X | X | Leaves, aerial parts | Hydrodistillation, Ethanol extract |
| Leonoruscardiaca L. [62] | X | Aerial parts | Hydroalcoholic extract | ||
| Leonotis leonorus L. [63] | X | X | Aerial parts | Organic extracts | |
| Leucasaspera Link [64] | X | X | Roots | Maceration | |
| Marrubiumvulgare. L. [65] | X | Leaves, aerial parts | Tincture, Organic extracts | ||
| Menthapiperita L. [66] | X | X | Leaves | Hydrodistillation | |
| M. suaveolens Ehrh. [67] | X | X | X | Aerial parts | Methanol extract |
| Ocimum americanum L. [68] | X | Aerial parts | Methanol extract | ||
| O. basilicum L. [69] | X | X | Aerial parts | Hydrodistillation | |
| Phlomispurpurea L. [70] | X | X | Aerial parts | Methanol extract | |
| P. nissolii L. [71] | X | Leaves | Decoction | ||
| Premna herbacea Roxb. [72] | X | X | Roots | Ethanol extract | |
| P. integrifolia L. [73] | X | Roots | Organic and aqueous extracts | ||
| Prunellavulgaris L. [74] | X | X | Inflorescence | Ethanol extract | |
| Rosmarinus officinalis L. [75,76] | X | X | X | Aerial parts, leaves | Maceration, Methanol extract |
| Salvia officinalis L. [77,78,79] | X | X | X | Aerial parts, Leaves | Infusion, Decoction, Hydroalcoholic extract |
| S. hispanica L. [80] | X | Aerial parts | Organic extracts | ||
| Scutellaria indica L. [81] | X | Aerial parts | Organic extracts | ||
| S. baicalensis Georgi. [82] | X | X | Aerial parts, Roots | Aqueous extract, Organic extract | |
| Sideritis bilgeriana P.H. Davis [83] | X | X | X | Aerial parts | Maceration |
| S. congesta P.H. Davis & Hub.-Mor. [84] | X | Aerial parts | Maceration | ||
| Stachysbyzantina C. Koch. [85] | X | X | Aerial parts | Organic extracts | |
| S. inflata Benth. [86] | X | Aerial parts | Hydroalcoholic extract | ||
| Thymusserpyllum L. [87] | X | Aerial parts | Hydrodistillation | ||
| T.vulgaris L. [88] | X | Leaves | Hydrodistillation | ||
| Vitexagnus-castus L. [89] | X | Leaves | Methanol extract | ||
| V. megapotamica Cham. [90] | X | X | Leaves | Hydroalcoholic extract | |
| Compound | Structure | Mechanism of Action | References |
|---|---|---|---|
| β-pinene |  | Decreased expression of IL-6, TNF-α, NO, iNOS and COX-2. Down-regulation of MAPKs phosphorylation and the NF-κB signaling pathway | [127] |
| Inhibition of COX-2 enzyme expression | [128] | ||
| Limonene |  | Reduction in leukocyte infiltration and TNF-α levels. | [129] |
| Decreased production of NO, PGE2 and Pro-inflammatory cytokines | [130] | ||
| Linalool |  | Inhibition of pro-inflammatory interleukins and modulation of NMDA glutamatergic receptor. | [131] |
| Reduction in oxidative stress and inflammation (NF-kB). | [132] | ||
| Activation of opioid and muscarinic receptors | [133] | ||
| Myrcene |  | Activation of opioid receptors and presynaptic α2 adrenoreceptor. | [134] |
| Inhibition of IL-1β-induced NO production Increased expression of TIMP-1 and TIMP-3. | [135] | ||
| p-cymene |  | Reduced the production of pro-inflammatory cytokine TNF-α, the migration of leukocytes, and the release of NO. Activation of opioid receptors. | [136] |
| Reduced the calcium current density. | [137] | ||
| Thymol |  | Voltage-operated sodium channel blocker | [138] |
| TRPA1 channel presynaptic activation | [139] | ||
| Carvacrol |  | Inhibition of expression TNF-α, IL-1β, and IL-6 Reduced the expression of NF-kB | [140] |
| Modulation of opioid, vanilloid and glutamate systems | [141] | ||
| α-humulene |  | Inhibition of pro-inflammatory cytokines (TNF-α and IL-1β) and PGE2 generation. Decreased expression of iNOS and COX-2. Inhibition of Il-5, CCL11 and LTB4 levels and P-selectin expression. | [142] [143] |
| β-caryophyllene |  | Cannabinoid receptor type 2 agonist. Attenuation of Substance P and cytokines such as IL-1β, TNF-α, and IL-6. | [144] |
| Agonist to opioid, benzodiazepine, 5HT1A receptors and NO. | [145] |
| Compound | Structure | Mechanism of Action | References |
|---|---|---|---|
| Tormentic acid |  | Inhibition of NF-kB signaling pathway and prevents the expressions of iNOS, COX-2, and TNF-α. | [152] |
| Increased activity of Superoxide dismutase, glutathione peroxidase and catalase. | [153] | ||
| Andalusol |  | Inhibition of histamine | [154] |
| Inhibition of iNOS expression by inactivation of NF-kB | [155] | ||
| Tanshinone IIA |  | TLR2/NF-kB signaling pathway blocker | [156] |
| Salvinorin A |  | KOR agonist. | [157] |
| Inhibition of dopamine overflow mediated by KOR. | [158] | ||
| α-amyrenone |  | PKC and PKA activity blocker. | [159] |
| Antioxidant activity. | [160] | ||
| β-amyrenone |  | Decreased levels of TNF-α and caspase 3 Reduction in oxidative stress. | [161] |
| Ursolic acid |  | NO, PGE2 inhibitor. | [162] |
| TRPV1 antagonist. Modulator of cGMP and serotonergic system. | [115] | ||
| Carnosol |  | Suppression of iNOS by down-regulation of NF-kB. | [163] |
| Suppression of PGE2 synthesis by the inhibition of mPGES-1. | [164] | ||
| Inhibition of the induction of COX-2 by blocking PKC signaling and thereby the binding of AP-1 to the CRE of the COX-2 promoter. | [165] | ||
| Oleanolic acid |  | Opioid agonist. NO inhibitor. Activation of ATP-gated K+ channels. | [166] |
| Opioid and 5-HT agonist. | [167] | ||
| Betulinic acid |  | Reduction in TNF-α production. Increase in IL-10 production. | [168] |
| Reduction in the levels of COX-2, NO, TNF-α and IL-1β. Inhibition of MDA level via increasing the activities of SOD, GPx, GRd. | [169] |
| Compound | Structure | Mechanisms of Action | References |
|---|---|---|---|
| Rosmarinic acid |  | Antioxidant activity. | [75] |
| Suppression of TNF-α, iNOs, apoptotic factors (Bax, caspases 3 and 9), Iba-1, TLR4 and GFAP levels. | [181] | ||
| Gallic acid |  | ERK-Nrf2-Keap1-mediated antioxidant activity. | [182] |
| Reduction in TBARS, total calcium, TNF-α, superoxide anion, and MPO activity levels; and decreased GSH level. | [183] | ||
| TRPA1 antagonist. | [184] | ||
| Chlorogenic acid |  | Inhibition of CD80/86 and Th1 cytokines. | [185] |
| GABAA receptor agonist. | [186] | ||
| Inhibition of NF-kB and JNK/AP-1 signaling pathways. | [187] | ||
| Vanillin |  | Inhibition of protein and lipid oxidation processes. Increased activity of GSH, SOD, catalase.Suppresses the expression of TNF-α, IL-6, IL-1β and plasma AST and ALT enzymes. | [188] |
| α2-adrenergic and opioid receptor agonist | [189] | ||
| Caffeic acid |  | Reduction in the IκBα degradation and p65 phosphorylation in the NF-kB pathway. | [190] |
| Inhibition of MPO, MDA and nitrite generation. | [191] | ||
| Vanillic acid |  | α2-adrenoceptor agonist.5HT3 and 5HT1 receptor agonist Interaction with TRPV1, TRPA1 and TRPM8 receptors. | [192] |
| Inhibition of oxidative stress, pro-inflammatory cytokine production, and NF-kB activation. | [193] | ||
| Ferulic acid |  | The level/activity of elastase, lysosomal enzymes, nitric oxide, lipid peroxidation, and pro-inflammatory cytokines (TNF-α and IL-1β); and the mRNA expression of NLRP3 inflammasomes, caspase-1, pro-inflammatory cytokines, and NF-kB p65 were decreased. | [194] |
| Inhibition of xanthine oxidase and COX-2 enzyme. | [195] |
| Compound | Structure | Mechanism of Action | References |
|---|---|---|---|
| Pedalitin |  | Inhibitory effects against NO, TNF-α and IL-12. | [202] |
| Rutin |  | Increased activity of GPx, GRd, CAT, SOD and GSH. | [203] |
| Central modulation of the vlPAG descending circuit partly mediated by an opioidergic mechanism. | [106] | ||
| Increased H2S level.Modulation of Nrf2 pathway. Caspase 3 and, NF-kB, TNF-α, IL-6 decreased.Increased sensory nerve conduction velocity. | [204] | ||
| Apigenin |  | Increased expression levels of Nrf2 and HO-1.Inhibition of TNF-α, IL-1β, IL-6, MPO and MDA content. | [24] |
| Inhibition of CD40, TNF-α and IL-6 | [205] | ||
| Quercetin |  | Interaction with L-arginine-nitric oxide, serotonin, and GABAergic systems. | [206] |
| ROCs and VOCs Blocker Modulation of PGF2α pathway | [207] | ||
| 5HT1A agonist | [208] | ||
| Luteolin |  | Inhibition of IL-1β, TNF-α and histamine release. | [209] |
| Decreased neutrophil infiltration.Inhibition of TNF-α, IL-1β, IL-6. | [210] | ||
| Downregulation of TLR4/TRAF6/NF-kB pathway | [211] | ||
| Inhibition of CD40, TNF-α and IL-6 | [205] | ||
| Hesperidin |  | Modulation of D2, GABAA and opioid receptors. | [212] |
| Agonist of opioid receptors. | [213] | ||
| Modulation of TRPV1 receptor. | [189] | ||
| Naringin |  | Inhibition of oxido-nitrosative strees, TNF-α, IL’s and NF-kB mRNA levels. | [214] |
| Inhibition of PGE2, NO, IL-6 and TNF-α. | [215] | ||
| Naringenin |  | Inhibition of NF-kB and activation of NO-Cyclic GMP-PKG-ATP sensitive K+ channel pathway | [216] |
| Inhibition of IL-6, TNF-α and NO release, by interfering MAPK signal pathway and suppressing the activation of NF-kB. | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Leon, A.; Moreno-Pérez, G.F.; Martínez-Gordillo, M.; Aguirre-Hernández, E.; Valle-Dorado, M.G.; Díaz-Reval, M.I.; González-Trujano, M.E.; Pellicer, F. Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief. Molecules 2021, 26, 7632. https://doi.org/10.3390/molecules26247632
Hernandez-Leon A, Moreno-Pérez GF, Martínez-Gordillo M, Aguirre-Hernández E, Valle-Dorado MG, Díaz-Reval MI, González-Trujano ME, Pellicer F. Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief. Molecules. 2021; 26(24):7632. https://doi.org/10.3390/molecules26247632
Chicago/Turabian StyleHernandez-Leon, Alberto, Gabriel Fernando Moreno-Pérez, Martha Martínez-Gordillo, Eva Aguirre-Hernández, María Guadalupe Valle-Dorado, María Irene Díaz-Reval, María Eva González-Trujano, and Francisco Pellicer. 2021. "Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief" Molecules 26, no. 24: 7632. https://doi.org/10.3390/molecules26247632
APA StyleHernandez-Leon, A., Moreno-Pérez, G. F., Martínez-Gordillo, M., Aguirre-Hernández, E., Valle-Dorado, M. G., Díaz-Reval, M. I., González-Trujano, M. E., & Pellicer, F. (2021). Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief. Molecules, 26(24), 7632. https://doi.org/10.3390/molecules26247632