Interactions of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside in the Presence and Absence of Iron during Thermal Processing and the Influence on Antioxidant Activity
Abstract
:1. Introduction
2. Results
2.1. Influence of Thermal Processing on Antioxidant Activity of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside Standards and the Mineral Iron
2.2. Influence of Iron and Different Combinations of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside Standards on Antioxidant Activity
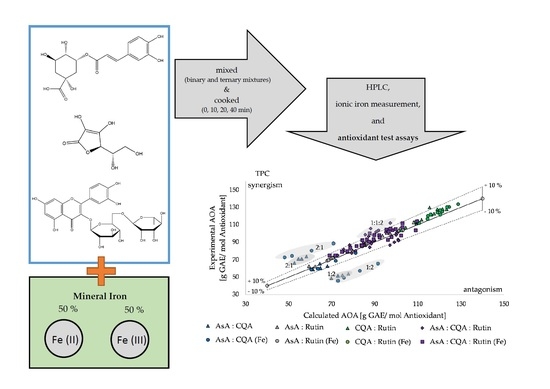
2.3. Synergistic and Antagonistic Effects of Antioxidant Activity
2.4. Total and Ionic iron
2.5. Qualitative and Quantitative Analysis of the Substance Mixtures by HPLC
3. Discussion
3.1. Structure–Activity Relationship of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside
3.2. Influence of Thermal Processing and Interaction of Structurally Different Antioxidants on the Antioxidant Activity in the Absence of the Mineral Iron
3.3. Influence of the Mineral Iron
3.4. Ability to Form Chelates with Ferric (Fe3+) and Ferrous Iron (Fe2+)
4. Materials and Methods
4.1. Chemicals
4.2. Samples
4.3. Photometric Measurements
4.3.1. Total Phenolic Content (TPC)
4.3.2. Trolox Equivalent Antioxidant Capacity (TEAC)
4.3.3. DPPH• Radical Scavenging
4.4. Synergism and Antagonism
4.5. Determination of Ionic Iron
4.6. HPLC-DAD
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mellor, D.D.; Naumovski, N. Effect of Cocoa in Diabetes: The Potential of the Pancreas and Liver as Key Target Organs, More than an Antioxidant Effect? Int. J. Food Sci. Technol. 2016, 51, 829–841. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 1–24. ISBN 1-4051-2509-8. [Google Scholar]
- Miller, D. Transition Metals as Catalysts of “Autoxidation” Reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of Thermal Processing on the Flavonols Rutin and Quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Iron Bioavailability in Humans from Breakfasts Enriched with Iron Bis-Glycine Chelate, Phytates and Polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of Non-Haem Iron Absorption in Man by Polyphenolic-Containing Beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I. Antiradical and Chelating Effects in Flavonoid Protection against Silica-Induced Cell Injury. Arch. Biochem. Biophys. 1998, 355, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Nowak, D.; Kuźniar, A.; Kopacz, M. Solid Complexes of Iron(II) and Iron(III) with Rutin. Struct. Chem. 2010, 21, 323–330. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The Biological Relevance of Direct Antioxidant Effects of Polyphenols for Cardiovascular Health in Humans Is Not Established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [Green Version]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [Green Version]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Zumreoglukaran, B. The Coordination Chemistry of Vitamin C: An Overview. Coord. Chem. Rev. 2006, 250, 2295–2307. [Google Scholar] [CrossRef]
- Hynes, M.J.; O’Coinceanainn, M. The Kinetics and Mechanisms of Reactions of Iron(III) with Caffeic Acid, Chlorogenic Acid, Sinapic Acid, Ferulic Acid and Naringin q. J. Inorg. Biochem. 2004, 98, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.V.; De Giovani, W.F. Antioxidant Properties of Complexes of Flavonoids with Metal Ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free Radical Scavenging and Antioxidant Activities of Favonoids Extracted from the Radix of Scutellaria Baicalensis Georgi. Biochim. Biophys. Acta BBA-Gen. Subj. 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant Properties of Flavonol Glycosides from Green Beans. Redox Rep. 1999, 4, 123–127. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zanoelo, E.F.; Benincá, C. Chemical Kinetics of 5-o-Caffeoylquinic Acid in Superheated Steam: Effect of Isomerization on Mate (Ilex paraguariensis) Manufacturing. J. Agric. Food Chem. 2009, 57, 11564–11569. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Typek, R. Thermal Stability of 5-o-Caffeoylquinic Acid in Aqueous Solutions at Different Heating Conditions. J. Agric. Food Chem. 2010, 58, 12578–12584. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L.; Samaniego, C.M.L. Effect of Initial Dissolved Oxygen Levels on the Degradation of Ascorbic Acid and the Browning of Lemon Juice during Storage. J. Food Sci. 1986, 51, 184–187. [Google Scholar] [CrossRef]
- Fellers, P.J. Shelf Life and Quality of Freshly Squeezed, Unpasteurized, Polyethylene-Bottled Citrus Juice. J. Food Sci. 1988, 53, 1699–1702. [Google Scholar] [CrossRef]
- Huelin, F.E. Studies on the Anaerobic Decomposition of Ascorbic Acid. J. Food Sci. 1953, 18, 633–639. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Shinoda, Y.; Murata, M.; Homma, S.; Komura, H. Browning and Decomposed Products of Model Orange Juice. Biosci. Biotechnol. Biochem. 2004, 68, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinoda, Y.; Komura, H.; Homma, S.; Murata, M. Browning of Model Orange Juice Solution: Factors Affecting the Formation of Decomposition Products. Biosci. Biotechnol. Biochem. 2005, 69, 2129–2137. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.-Y.; Tsai, Y.-C.; Fu, C.-C.; Wu, J.S.-B. Degradation of Ascorbic Acid in Ethanolic Solutions. J. Agric. Food Chem. 2012, 60, 10696–10701. [Google Scholar] [CrossRef]
- Hassan, S.; Adam, F.; Abu Bakar, M.R.; Abdul Mudalip, S.K. Evaluation of Solvents’ Effect on Solubility, Intermolecular Interaction Energies and Habit of Ascorbic Acid Crystals. J. Saudi Chem. Soc. 2019, 23, 239–248. [Google Scholar] [CrossRef]
- Saucier, C.T.; Waterhouse, A.L. Synergetic Activity of Catechin and Other Antioxidants. J. Agric. Food Chem. 1999, 47, 4491–4494. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, L.M.; Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Waksmundzka-Hajnos, M. Antioxidant Synergism and Antagonism between Selected Monoterpenes Using the 2,2-Diphenyl-1-Picrylhydrazyl Method: Antioxidant Synergism and Antagonism between Selected Monoterpenes. Flavour Fragr. J. 2016, 31, 412–419. [Google Scholar] [CrossRef]
- Boligon, A.A. Technical Evaluation of Antioxidant Activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Bolling, B.W.; Chen, Y.-Y.; Chen, C.-Y.O. Contributions of Phenolics and Added Vitamin C to the Antioxidant Capacity of Pomegranate and Grape Juices: Synergism and Antagonism among Constituents. Int. J. Food Sci. Technol. 2013, 48, 2650–2658. [Google Scholar] [CrossRef]
- Bravo, A.; Anacona, J.R. Metal Complexes of the Favonoid Quercetin: Antibacterial Properties. Transit. Met. Chem. 2001, 26, 20–23. [Google Scholar] [CrossRef]
- van Acker, S.A.B.E.; van Balen, G.P.; van den Berg, D.; Bast, A.; van der Vijgh, W.J.F. Influence of Iron Chelation on the Antioxidant Activity of Flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef]
- Symonowicz, M.; Kolanek, M. Flavonoids and Their Properties to Form Chelate Complexes. Biotechnol. Food Sci. 2012, 76, 35–41. [Google Scholar]
- Kopjar, M.; Jakšić, K.; Piližota, V. Influence of Sugars and Chlorogenic Acid Addition on Anthocyanin Content, Antioxidant Activity and Color of Blackberry Juice during Storage: Anthocyanin Content of Blackberry Juice. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Iwahashi, H.; Ishii, T.; Sugata, R.; Kido, R. The Effects of Caffeic Acid and Its Related Catechols on Hydroxyl Radical Formation by 3-Hydroxyanthranilic Acid, Ferric Chloride, and Hydrogen Peroxide. Arch. Biochem. Biophys. 1990, 276, 242–247. [Google Scholar] [CrossRef]
- Rao, C.P.; Geetha, K.; Raghavan, M.S.S.; Sreedhara, A.; Tokunaga, K.; Yamaguchi, T.; Jadhav, V.; Ganesh, K.N.; Krishnamoorthy, T.; Ramaiah, K.V.A.; et al. Transition Metal Saccharide Chemistry and Biology: Syntheses, Characterization, Solution Stability and Putative Bio-Relevant Studies of Iron–Saccharide Complexes. Inorg. Chim. Acta 2000, 297, 373–382. [Google Scholar] [CrossRef]
- Uranga, J.G.; Podio, N.S.; Wunderlin, D.A.; Santiago, A.N. Theoretical and Experimental Study of the Antioxidant Behaviors of 5-O-Caffeoylquinic, Quinic and Caffeic Acids Based on Electronic and Structural Properties. ChemistrySelect 2016, 1, 4113–4120. [Google Scholar] [CrossRef]
- Deiana, S.; Gessa, C.; Manunza, B.; Rausa, R.; Solinas, V. Iron(III) Reduction by Natural Humic Acids: A Potentiometric and Spectroscopic Study. Eur. J. Soil Sci. 1995, 46, 103–108. [Google Scholar] [CrossRef]
- Avdeef, A.; Sofen, S.R.; Bregante, T.L.; Raymond, K.N. Coordination Chemistry of Microbial Iron Transport Compounds. 9. Stability Constants for Catechol Models of Enterobactin. J. Am. Chem. Soc. 1978, 100, 5362–5370. [Google Scholar] [CrossRef]
- Lamy, I.; Seywert, M.; Cromer, M.; Scharff, J.-P. Simple and Mixed Ligand Complexes of Copper (II) with Polyfunctional Phenolic Compounds as Models of Natural Substances. Anal. Chim. Acta 1985, 176, 201–212. [Google Scholar] [CrossRef]
- Kiss, T.; Nagy, G.; Pécsi, M.; Kozlowski, H.; Micera, G.; Erre, L.S. Complexes of 3,4-Dihydroxyphenyl Derivatives—X. Copper(II) Complexes of Chlorogenic Acid and Related Compounds. Polyhedron 1989, 8, 2345–2349. [Google Scholar] [CrossRef]
- Améziane, J.; Aplincourt, M.; Dupont, L.; Heirman, F.; Pierrard, J.-C. Thermodynamic Stability of Copper (II), Manganese (II), Zinc (II) and Iron (II) Complexes with Chlorogenic Acid. Bull. Soc. Chim. Fr. 1996, 133, 243–249. [Google Scholar]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron Chelation by Chlorogenic Acid as a Natural Antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for Their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods: Comparison of Microplate and Conventional Methods for Folin- Ciocalteu and DPPH. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Investigation of Antioxidant Activity of Selenium Compounds and Their Mixtures with Tea Polyphenols. Mol. Biol. Rep. 2019, 46, 3019–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzielski, P.; Zielinska-Dawidziak, M.; Kozak, L.; Kowalewski, P.; Szlachetka, B.; Zalicka, S.; Wachowiak, W. Determination of Iron Species in Samples of Iron-Fortified Food. Food Anal. Methods 2014, 7, 2023–2032. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R package version 1.5.3. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 11 December 2021).
- Hothorn, T.; Brenz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Substance | Time [min] | Ferrous Iron [%] | Ferric Iron [%] a | Bound Iron [%] * |
|---|---|---|---|---|
| Ascorbic acid (AsA) | 0 | 77.9 ± 0.8 | traces | 21.17 ± 0.4 |
| 10 | 98.9 ± 0.8 | traces | traces | |
| 20 | 97.1 ± 0.9 | traces | traces | |
| 40 | 96.7 ± 1.1 | traces | traces | |
| 5-Caffeoylquinic acid (CQA) | 0 | 57.73 ± 3.8 | 42.27 ± 3.8 | - |
| 10 | 64.04 ± 4.2 | 35.96 ± 4.2 | - | |
| 20 | 70.51 ± 3.3 | 26.78 ± 2.1 | traces | |
| 40 | 67.80 ± 2.4 | 28.97 ± 4.9 | traces | |
| Quercetin-3-rutinoside (Rutin) | 0 | 55.02 ± 2.1 | 44.98 ± 2.1 | - |
| 10 | 55.25 ± 3.2 | 42.20 ± 3.7 | traces | |
| 20 | 56.65 ±4.3 | 27.38 ± 5.2 | 15.97 ± 2.1 | |
| 40 | 54.77 ±4.2 | 24.49 ± 4.3 | 20.74 ± 8.3 |
| Substance | Time [min] | Ferrous Iron [%] | Ferric Iron [%] a | Bound Iron [%] * |
|---|---|---|---|---|
| 1 AsA: 1 CQA | 0 | 68.6 ± 0.8 | traces | 28.9 ± 0.2 |
| 10 | 97.4 ± 0.7 | traces | traces | |
| 20 | 94.1 ± 0.4 | 5.5 ± 1.0 | traces | |
| 40 | 92.6 ± 2.6 | 7.4 ± 2.6 | - | |
| 1 AsA: 2 CQA | 0 | 73.2 ± 8.7 | 5.4 ± 1.0 | 21.4 ± 7.8 |
| 10 | 91.2 ± 0.5 | 8.8 ± 0.5 | - | |
| 20 | 92.0 ± 0.8 | 8.0 ± 0.8 | - | |
| 40 | 91.0 ± 2.1 | 8.6 ± 2.4 | traces | |
| 2 AsA: 1 CQA | 0 | 79.5 ± 11.5 | traces | 18.9 ± 11.2 |
| 10 | 98.8 ± 0.8 | traces | traces | |
| 20 | 96.5 ± 0.7 | traces | traces | |
| 40 | 93.9 ± 0.8 | 5.8 ± 0.9 | traces | |
| 1 AsA: 1 Rutin | 0 | 74.5 ± 7.7 | traces | 23.1 ± 7.1 |
| 10 | 90.9 ± 3.0 | 9.1 ± 3.0 | - | |
| 20 | 86.4 ± 0.2 | 13.6 ± 0.2 | - | |
| 40 | 81.1 ± 5.7 | 18.8 ± 5.7 | traces | |
| 1 AsA: 2 Rutin | 0 | 73.0 ± 7.0 | 5.9 ± 1.4 | 21.1 ± 6.0 |
| 10 | 80.8 ± 1.3 | 19.2 ± 1.3 | - | |
| 20 | 79.3 ± 0.6 | 20.7 ± 0.6 | - | |
| 40 | 76.7 ± 4.5 | 21.8 ± 6.2 | traces | |
| 2 AsA: 1 Rutin | 0 | 76.2 ± 9.1 | traces | 22.0 ± 8.7 |
| 10 | 96.5 ± 2.3 | traces | traces | |
| 20 | 89.7 ± 0.1 | 10.3 ± 0.1 | - | |
| 40 | 84.9 ± 3.5 | 15.1 ± 3.5 | - | |
| 1 CQA: 1 Rutin | 0 | 56.6 ± 1.8 | 43.4 ± 1.8 | - |
| 10 | 60.4 ± 3.8 | 38.3 ± 3.8 | traces | |
| 20 | 65.0 ± 4.0 | 29.4 ± 2.2 | 5.6 ± 3.2 | |
| 40 | 61.6 ± 1.2 | 33.1 ± 5.4 | 5.3 ± 4.3 | |
| 1 CQA: 2 Rutin | 0 | 55.2 ± 2.4 | 44.8 ± 2.4 | - |
| 10 | 59.4 ± 4.0 | 39.7 ± 3.8 | traces | |
| 20 | 63.3 ± 3.9 | 28.9 ± 3.3 | 7.8 ± 5.0 | |
| 40 | 60.7 ± 3.0 | 30.5 ± 3.7 | 8.8 ± 6.6 | |
| 2 CQA: 1 Rutin | 0 | 56.1 ± 3.2 | 43.9 ± 3.2 | - |
| 10 | 61.4 ± 3.6 | 38.4 ± 3.5 | traces | |
| 20 | 66.5 ± 3.9 | 26.9 ± 1.3 | 6.6 ± 2.6 | |
| 40 | 62.8 ± 3.2 | 29.5 ± 8.0 | 7.7 ± 5.6 |
| Substance | Time [min] | Ferrous Iron [%] | Ferric Iron [%] a | Bound Iron [%] * |
|---|---|---|---|---|
| 1 AsA: 1 CQA: 1 Rutin | 0 | 69.65 ± 7.8 | 6.94 ± 1.3 | 23.41 ± 7.0 |
| 10 | 84.66 ± 1.2 | 15.34 ± 1.2 | - | |
| 20 | 84.10 ± 1.2 | 15.90 ± 1.2 | - | |
| 40 | 81.39 ± 3.3 | 16.30 ± 4.9 | traces | |
| 1 AsA: 2 CQA: 1 Rutin | 0 | 69.16 ± 6.2 | 12.04 ± 0.4 | 18.80 ± 6.3 |
| 10 | 79.05 ± 0.8 | 20.95 ± 0.8 | - | |
| 20 | 78.93 ± 2.8 | 20.53 ± 2.5 | traces | |
| 40 | 74.91 ± 4.9 | 22.90 ± 6.5 | traces | |
| 1 AsA: 1 CQA: 2 Rutin | 0 | 69.62 ± 6.5 | 12.47 ± 0.5 | 17.91 ± 6.2 |
| 10 | 77.88 ± 0.8 | 22.12 ± 0.8 | - | |
| 20 | 78.05 ± 0.6 | 21.25 ± 1.4 | traces | |
| 40 | 71.91 ± 5.6 | 25.41 ± 7.5 | traces | |
| 2 AsA: 1 CQA: 1 Rutin | 0 | 73.37 ± 8.9 | traces | 23.87 ± 8.3 |
| 10 | 90.88 ± 2.1 | 9.12 ± 2.1 | - | |
| 20 | 88.78 ± 0.2 | 11.22 ± 0.2 | - | |
| 40 | 87.75 ± 0.7 | 12.25 ± 0.7 | - | |
| 1 AsA: 2 CQA: 2 Rutin | 0 | 67.85 ± 6.3 | 18.60 ± 0.8 | 13.55 ± 5.5 |
| 10 | 74.33 ± 0.8 | 25.67 ± 0.8 | - | |
| 20 | 74.51 ± 1.0 | 23.40 ± 0.5 | traces | |
| 40 | 69.98 ± 4.3 | 24.64 ± 8.1 | 5.38 ± 3.8 | |
| 2 AsA: 1 CQA: 2 Rutin | 0 | 71.34 ± 8.3 | 5.34 ± 1.3 | 23.32 ± 7.5 |
| 10 | 87.63 ± 2.0 | 12.37 ± 2.0 | - | |
| 20 | 86.47 ± 0.5 | 13.25 ± 0.6 | traces | |
| 40 | 82.40 ± 4.1 | 17.36 ± 4.3 | traces | |
| 2 AsA: 2 CQA: 1 Rutin | 0 | 71.62 ± 0.8 | 5.11 ± 1.0 | 23.28 ± 7.3 |
| 10 | 88.93 ± 0.3 | 11.07 ± 0.3 | - | |
| 20 | 89.31 ± 1.1 | 9.85 ± 2.0 | traces | |
| 40 | 85.34 ± 3.6 | 14.50 ± 3.8 | traces |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelhardt, L.; Pöhnl, T.; Neugart, S. Interactions of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside in the Presence and Absence of Iron during Thermal Processing and the Influence on Antioxidant Activity. Molecules 2021, 26, 7698. https://doi.org/10.3390/molecules26247698
Engelhardt L, Pöhnl T, Neugart S. Interactions of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside in the Presence and Absence of Iron during Thermal Processing and the Influence on Antioxidant Activity. Molecules. 2021; 26(24):7698. https://doi.org/10.3390/molecules26247698
Chicago/Turabian StyleEngelhardt, Layla, Tobias Pöhnl, and Susanne Neugart. 2021. "Interactions of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside in the Presence and Absence of Iron during Thermal Processing and the Influence on Antioxidant Activity" Molecules 26, no. 24: 7698. https://doi.org/10.3390/molecules26247698
APA StyleEngelhardt, L., Pöhnl, T., & Neugart, S. (2021). Interactions of Ascorbic Acid, 5-Caffeoylquinic Acid, and Quercetin-3-Rutinoside in the Presence and Absence of Iron during Thermal Processing and the Influence on Antioxidant Activity. Molecules, 26(24), 7698. https://doi.org/10.3390/molecules26247698