Nitro-Substituted Dipyrrolyldiketone BF2 Complexes as Electronic-State-Adjustable Anion-Responsive π-Electronic Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Photophysical Properties
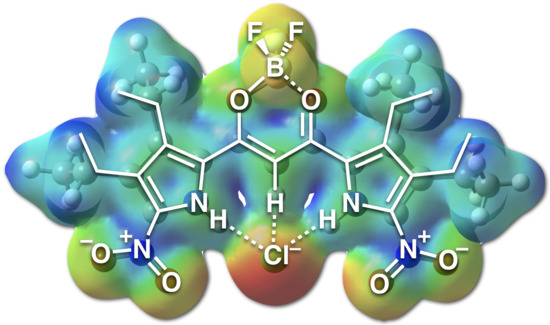
2.3. Anion-Binding Behavior
3. Materials and Methods
3.1. General Procedures
3.1.1. BF2 Complex of 1,3-bis(3,4-diethyl-5-nitropyrrol-2-yl)-1,3-propanedione, 2a
3.1.2. BF2 Complex of 1-(3,4-diethyl-5-nitropyrrol-2-yl)-3-(3,4-diethylpyrrol-2-yl)-1,3-propanedione, 2b
3.1.3. BF2 Complex of 3-(3,4-diethyl-5-iodopyrrol-2-yl)-1-(3,4-diethyl-5-nitropyrrol-2-yl)-1,3-propanedione, 2c
3.2. Method for Single-Crystal X-ray Analysis
3.3. DFT Caluculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Self-Assembly and Supramolecular Devices. In Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P.A.; Steed, J.W. (Eds.) Wiley: Hoboken, NJ, USA, 2012; Volume 5. [Google Scholar]
- Kokado, K.; Sada, K. Consideration of Molecular Structure in the Excited State to Design New Luminogens with Aggregation-Induced Emission. Angew. Chem. Int. Ed. 2019, 58, 8632–8639. [Google Scholar] [CrossRef] [PubMed]
- Haupt, A.; Lentz, D. Corannulenes with Electron-Withdrawing Substituents: Synthetic Approaches and Resulting Structural and Electronic Properties. Chem. Eur. J. 2019, 25, 3440–3454. [Google Scholar] [CrossRef] [PubMed]
- Cherumukkil, S.; Vedhanarayanan, B.; Das, G.; Praveen, V.K.; Ajayaghosh, A. Self-Assembly of Bodipy-Derived Extended π-Systems. Bull. Chem. Soc. Jpn. 2018, 91, 100–120. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor–acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- An, B.-K.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K. π-Organogels of Self-Assembled p-Phenylenevinylenes: Soft Materials with Distinct Size, Shape, and Functions. Acc. Chem. Res. 2007, 40, 644–656. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Chen, M.-C.; Chen, D.-G.; Chou, P.-T. Fluorescent Chromophores Containing the Nitro Group: Relatively Unexplored Emissive Properties. ChemPlusChem 2021, 86, 11–27. [Google Scholar] [CrossRef]
- Ueta, K.; Tanaka, T.; Osuka, A. Synthesis and Characterizations of meso-Nitrocorroles. Chem. Lett. 2018, 47, 916–919. [Google Scholar] [CrossRef]
- Poronik, Y.M.; Baryshnikov, G.V.; Deperasińska, I.; Espinoza, E.M.; Clark, J.A.; Ågren, H.; Gryko, D.T.; Vullev, V.I. Deciphering the unusual fluorescence in weakly coupled bis-nitro-pyrrolo[3,2-b]pyrroles. Commun. Chem. 2020, 3, 190. [Google Scholar] [CrossRef]
- Yadav, M.R.; Nagaoka, M.; Kashihara, M.; Zhong, R.-L.; Miyazaki, T.; Sakaki, S.; Nakao, Y. The Suzuki–Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc. 2017, 139, 9423–9426. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kusunose, Y. Dipyrrolyldiketone Difluoroboron Complexes: Novel Anion Sensors with C-H···X– Interactions. Chem. Eur. J. 2005, 11, 5661–5666. [Google Scholar] [CrossRef]
- Maeda, H.; Ito, Y. BF2 Complex of Fluorinated Dipyrrolyldiketone: A New Class of Efficient Receptor for Acetate Anions. Inorg. Chem. 2006, 45, 8205–8210. [Google Scholar] [CrossRef]
- Maeda, H.; Kusunose, Y.; Mihashi, Y.; Mizoguchi, T. BF2 Complexes of β-Tetraethyl-Substituted Dipyrrolyldiketones as Anion Receptors: Potential Building Subunits for Oligomeric Systems. J. Org. Chem. 2007, 72, 2612–2616. [Google Scholar] [CrossRef]
- Maeda, H.; Haketa, Y. Selective iodinated dipyrrolyldiketone BF2 complexes as potential building units for oligomeric systems. Org. Biomol. Chem. 2008, 6, 3091–3095. [Google Scholar] [CrossRef]
- Yamakado, R.; Sakurai, T.; Matsuda, W.; Seki, S.; Yasuda, N.; Akine, S.; Maeda, H. π-Electronic Systems That Form Planar and Interlocked Anion Complexes and Their Ion-Pairing Assemblies. Chem. Eur. J. 2016, 22, 626–638. [Google Scholar] [CrossRef]
- Haketa, Y.; Maeda, H. Dimension-Controlled π-Electronic Ion-Pairing Assemblies. Bull. Chem. Soc. Jpn. 2018, 91, 420–436. [Google Scholar] [CrossRef]
- Haketa, Y.; Urakawa, K.; Maeda, H. First decade of π-electronic ion-pairing assemblies. Mol. Syst. Des. Eng. 2020, 5, 757–771. [Google Scholar] [CrossRef]
- Pantoş, G.D.; Rodríguez-Morgade, M.S.; Torres, T.; Lyncha, V.M.; Sessler, J.L. 2-Amino-3,4-diethylpyrrole derivatives: New building blocks for coiled structures. Chem. Commun. 2006, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Appendix A. Properties Purification and Use of Organic Solvents. In Solvents and Solvent Effects in Organic Chemistry; Reichardt, C.; Welton, T. (Eds.) Wiley: Hoboken, NJ, USA, 2010; pp. 549–586. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Wakita, K. Yadokari-XG, Software for Crystal Structure Analyses. 2001. Available online: http://chem.s.kanazawa-u.ac.jp/coord/yadokari/y-citation.html (accessed on 6 January 2021).
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of software (Yadokari-XG 2009) for crystal structure analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]









| 2a | 2b | 1b | |
|---|---|---|---|
| Cl− | 190,000 | 27,000 | 6800 |
| Br− | 11,000 | 2800 | 1200 |
| CH3CO2− | >106 | >106 | 210,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuno, A.; Maeda, H. Nitro-Substituted Dipyrrolyldiketone BF2 Complexes as Electronic-State-Adjustable Anion-Responsive π-Electronic Systems. Molecules 2021, 26, 595. https://doi.org/10.3390/molecules26030595
Kuno A, Maeda H. Nitro-Substituted Dipyrrolyldiketone BF2 Complexes as Electronic-State-Adjustable Anion-Responsive π-Electronic Systems. Molecules. 2021; 26(3):595. https://doi.org/10.3390/molecules26030595
Chicago/Turabian StyleKuno, Atsuko, and Hiromitsu Maeda. 2021. "Nitro-Substituted Dipyrrolyldiketone BF2 Complexes as Electronic-State-Adjustable Anion-Responsive π-Electronic Systems" Molecules 26, no. 3: 595. https://doi.org/10.3390/molecules26030595
APA StyleKuno, A., & Maeda, H. (2021). Nitro-Substituted Dipyrrolyldiketone BF2 Complexes as Electronic-State-Adjustable Anion-Responsive π-Electronic Systems. Molecules, 26(3), 595. https://doi.org/10.3390/molecules26030595