Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
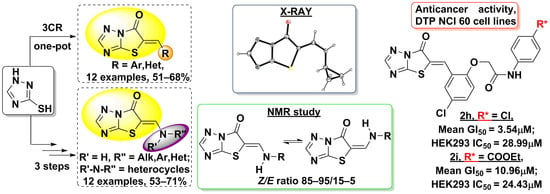
2.1. Chemical Synthesis
2.2. Spectral Characterization
2.3. The X-ray Crystal Structure Analysis of (Z)-5-Cyclopropylaminomethylidene-[1,3]thiazolo-[3,2-b][1,2,4]triazol-6(5H)-one (5c)
2.4. In Vitro Evaluation of the Anticancer Activity and Cytotoxicity. Compare Analysis
2.5. The Preliminary Structure-Anticancer Activity Relationship for Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones
3. Materials and Methods
3.1. General Information
3.2. Preparation and Characterization of Compounds
3.2.1. Characterization of Compounds 2a–l
3.2.2. Synthesis of 2-((1H-1,2,4-Triazol-3-yl)thio)acetic Acid (3)
3.2.3. Synthesis and Characterization of Compounds 5a–h, 6a–d
3.3. Crystal Structure Determination of (E/Z)-5-((Cyclopropylamino)methylenethiazolo-[3,2-b]-[1,2,4]-triazol-6(5H)-one (5c)
3.3.1. Crystal Data
3.3.2. Data Collection
3.3.3. Structure Solution and Refinement
3.4. Anticancer Activity Screening (NCI-60 Human Tumor Cell Lines Screen)
3.5. Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- The global challenge of cancer. Nat. Cancer 2020, 1, 1–2. [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother 2020, 25, 422–427. [Google Scholar] [CrossRef]
- Čipak Gašparović, A. Free Radical Research in Cancer. Antioxidants (Basel) 2020, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherkas, A.; Zarkovic, N. 4-Hydroxynonenal in Redox Homeostasis of Gastrointestinal Mucosa: Implications for the Stomach in Health and Diseases. Antioxidants (Basel) 2018, 7, 118. [Google Scholar] [CrossRef]
- Deng, X.; Nakamura, Y. Cancer Precision Medicine: From Cancer Screening to Drug Selection and Personalized Immunotherapy. Trends Pharmacol. Sci. 2017, 38, 15–24. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Saso, L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox. Biol. 2017, 12, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants (Basel) 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Slivka, M.V.; Korol, N.I.; Fizer, M.M. Fused bicyclic 1,2,4-triazoles with one extra sulfur atom: Synthesis, properties, and biological activity. J. Heterocycl Chem 2020, 57, 3236–3254. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Gökhan, N.; Aktay, G.; Yeşilada, E.; Ertan, M. 6-Benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6H)-ones substituted with ibuprofen: Synthesis, characterization and evaluation of anti-inflammatory activity. Eur. J. Med. Chem. 2000, 35, 743–750. [Google Scholar] [CrossRef]
- Assarzadeh, M.J.; Almasirad, A.; Shafiee, A.; Koopaei, M.N.; Abdollahi, M. Synthesis of new thiazolo[3,2-b][1,2,4]triazole-6(5H)-one derivatives as potent analgesic and anti-inflammatory agents. Med. Chem. Res. 2014, 23, 948–957. [Google Scholar] [CrossRef]
- Toma, A.; Mogoşan, C.; Vlase, L.; Leonte, D.; Zaharia, V. Heterocycles 39. Synthesis, characterization and evaluation of the anti-inflammatory activity of thiazolo[3,2-b][1,2,4]triazole derivatives bearing pyridin-3/4-yl moiety. Med. Chem. Res. 2017, 26, 2602–2613. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Gökhan, N.; Küpeli, E.; Yeşilada, E.; Ertan, M. Synthesis, characterization and antiinflammatory-analgesic properties of 6-(alpha-amino-4-chlorobenzyl)thiazolo[3,2-b]-1,2,4-triazol-5-ols. Arzneimittelforschung 2004, 54, 35–41. [Google Scholar] [CrossRef]
- Tratrat, C.; Haroun, M.; Paparisva, A.; Kamoutsis, C.; Petrou, A.; Gavalas, A.; Eleftheriou, P.; Geronikaki, A.; Venugopala, K.N.; Kochkar, H.; et al. New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents. Molecules 2021, 26, 659. [Google Scholar] [CrossRef] [PubMed]
- Barbuceanu, S.F.; Draghici, C.; Barbuceanu, F.; Bancescu, G.; Saramet, G. Design, Synthesis, Characterization and Antimicrobial Evaluation of Some Heterocyclic Condensed Systems with Bridgehead Nitrogen from Thiazolotriazole Class. Chem. Pharm. Bull. (Tokyo) 2015, 63, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Tratrat, C.; Haroun, M.; Paparisva, A.; Geronikaki, A.; Kamoutsis, Ch.; Ćirić, A.; Glamočlija, J.; Soković, M.; Fotakis, Ch.; Zoumpoulakis, P.; et al. Design, synthesis and biological evaluation of new substituted 5-benzylideno-2-adamantylthiazol[3,2-b][1,2,4]triazol-6(5H)ones. Pharmacophore models for antifungal activity. Arab. J. Chem. 2018, 11, 573–590. [Google Scholar] [CrossRef] [Green Version]
- Djukic, M.; Fesatidou, M.; Xenikakis, I.; Geronikaki, A.; Angelova, V.T.; Savic, V.; Pasic, M.; Krilovic, B.; Djukic, D.; Gobeljic, B.; et al. In vitro antioxidant activity of thiazolidinone derivatives of 1,3-thiazole and 1,3,4-thiadiazole. Chem. Biol. Interact. 2018, 286, 119–131. [Google Scholar] [CrossRef]
- Vijaya Raj, K.K.; Narayana, B. The One Step Synthesis of 2-(2-Bromo-5-methoxyphenyl)-5-(3-arylidene)-1,3-thiazolo[3,2-b]-1,2,4-triazol-6-(5H)-ones and the Evaluation of the Anticonvulsant Activity. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 1971–1981. [Google Scholar] [CrossRef]
- Deng, X.Q.; Song, M.X.; Gong, G.H.; Wang, S.B.; Quan, Z.S. Synthesis and Anticonvulsant Evaluation of some New 6-(Substituted-phenyl)thiazolo[3,2-b][1,2,4]triazole Derivatives in Mice. Iran. J. Pharm. Res. 2014, 13, 459–469. [Google Scholar] [PubMed]
- Foks, H.; Czarnocka-Janowicz, A.; Rudnicka, W.; Damasiewicz, B.; Nasal, A. Synthesis and biological activity of thiazolo-1,2,4-triazoles. Acta Pol. Pharm. 1995, 52, 415–420. [Google Scholar] [PubMed]
- Tozkoparan, B.; Akgün, H.; Ertan, M.; Rübsemann, K. Synthesis of some thiazolo[3,2-b]-1,2,4-triazole-5(6H)-ones as potential platelet aggregation inhibitors. Arch. Pharm. (Weinheim) 1995, 328, 169–173. [Google Scholar] [CrossRef]
- Berk, B.; Aktay, G.; Yesilada, E.; Ertan, M. Synthesis and pharmacological activities of some new 2-[1-(6-methoxy-2-naphthyl)ethyl]-6-(substituted)benzylidene thiazolo[3,2-b]-1,2,4-triazole-5(6H)-one derivatives. Pharmazie 2001, 56, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Doğdaş, E.; Tozkoparan, B.; Kaynak, F.B.; Eriksson, L.; Küpeli, E.; Yeşilada, E.; Ertan, M. Design and synthesis of some new thiazolo[3,2-b]-1,2,4-triazole-5(6H)-ones substituted with flurbiprofen as anti-inflammatory and analgesic agents. Arzneimittelforschung 2007, 57, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Uzgören-Baran, A.; Tel, B.C.; Sarıgöl, D.; Oztürk, E.İ.; Kazkayası, I.; Okay, G.; Ertan, M.; Tozkoparan, B. Thiazolo[3,2-b]-1,2,4-triazole-5(6H)-one substituted with ibuprofen: Novel non-steroidal anti-inflammatory agents with favorable gastrointestinal tolerance. Eur. J. Med. Chem. 2012, 57, 398–406. [Google Scholar] [CrossRef]
- Dong, G.; Sheng, C.; Wang, S.; Miao, Z.; Yao, J.; Zhang, W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 2010, 53, 7521–7531. [Google Scholar] [CrossRef]
- Sheng, C.; Miao, Z.; Zhang, W. New strategies in the discovery of novel non-camptothecin topoisomerase I inhibitors. Curr. Med. Chem. 2011, 18, 4389–4409. [Google Scholar] [CrossRef]
- Reynisson, J.; Court, W.; O’Neill, C.; Day, J.; Patterson, L.; McDonald, E.; Workman, P.; Katan, M.; Eccles, S.A. The identification of novel PLC-gamma inhibitors using virtual high throughput screening. Bioorg. Med. Chem. 2009, 17, 3169–3176. [Google Scholar] [CrossRef]
- Eurtivong, C.; Pilkington, L.I.; van Rensburg, M.; White, R.M.; Brar, H.K.; Rees, S.; Paulin, E.K.; Xu, C.S.; Sharma, N.; Leung, I.K.H.; et al. Discovery of novel phosphatidylcholine-specific phospholipase C drug-like inhibitors as potential anticancer agents. Eur. J. Med. Chem. 2020, 187, 111919. [Google Scholar] [CrossRef]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef]
- Feng, L.; Reynisdóttir, I.; Reynisson, J. The effect of PLC-γ2 inhibitors on the growth of human tumour cells. Eur. J. Med. Chem. 2012, 54, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Holota, S.M.; Derkach, H.O.; Demchuk, I.L.; Vynnytska, R.B.; Antoniv, O.I.; Furdychko, L.O.; Slyvka, N.Yu.; Nektegayev, I.O.; Lesyk, R.B. Synthesis and in vivo evaluation of pyrazoline-thiazolidin-4-one hybrid Les-5581 as a potential non-steroidal anti-inflammatory agent. Biopolym. Cell 2019, 35, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Holota, S.M.; Derkach, G.O.; Zasidko, V.V.; Trokhymchuk, V.V.; Furdychko, L.O.; Demchuk, I.L.; Semenciv, G.M.; Soronovych, I.I.; Kutsyk, R.V.; Lesyk, R.B. Features of antimicrobial activity of some 5-aminomethylene-2-thioxo-4-thiazolidinones. Biopolym. Cell 2019, 35, 371–380. [Google Scholar] [CrossRef]
- Schadich, E.; Kryshchyshyn-Dylevych, A.; Holota, S.; Polishchuk, P.; Džubak, P.; Gurska, S.; Hajduch, M.; Lesyk, R. Assessing different thiazolidine and thiazole based compounds as antileishmanial scaffolds. Bioorg. Med. Chem. Lett. 2020, 30, 127616. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Holota, S.; Yushyn, I.; Sabadakh, O.; Karpenko, O.; Novikov, V.; Lesyk, R. Synthesis and Biological Activity Evaluation of Polyfunctionalized Anthraquinonehydrazones. Lett. Drug Des. Discov. 2021, 18, 2. [Google Scholar] [CrossRef]
- Holota, S.; Shylych, Ya.; Derkach, H.; Karpenko, O.; Gzella, A.; Lesyk, R. Synthesis of 4-(2H-[1,2,4]-Triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one via Ring-Switching Hydrazinolysis of 5-Ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one. Molbank 2018, 4, M1022. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin. Trans. 1987, 2, S1–19. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some Practical Considerations and Applications of the National Cancer Institute in Vitro Anticancer Drug Discovery Screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 2, pp. 23–42. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Holbeck, S.L. Update on NCI in vitro drug screen utilities. Eur. J. Cancer 2004, 40, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Cancer Ther. 2010, 9, 1451–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grange, E.W.; Henry, D.W.; Lee, W.W. Glyoxylic Acid Hydrocarbylsulfonylhydrazones and Therapeutic Compositions. U.S. Patent US4218465A, 19 August 1980. [Google Scholar]
- Pretzer, D.; Repta, A.J. Stability of the experimental anticancer agent [[(4-methoxyphenyl)sulfonyl]hydrazono]acetic acid (NSC-267213). I. Kinetics and mechanism of degradation. Int. J. Pharm. 1987, 38, 147–160. [Google Scholar] [CrossRef]
- Pretzer, D.; Repta, A.J. Stability of the experimental anticancer agent [[(4-methoxyphenyl) sulfonyl] hydrazono] acetic acid (NSC-267213). II. Effect of structural modification on stability and mechanism of degradation. Int. J. Pharm. 1987, 38, 161–169. [Google Scholar] [CrossRef]
- Shyam, K.; Cosby, L.A.; Sartorelli, A.C. Relationship between structure and antineoplastic activity of (arylsulfonyl)hydrazones of 4-pyridinecarboxaldehyde. J. Med. Chem. 1985, 28, 149–152. [Google Scholar] [CrossRef]
- Morgan, D.M. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 1998, 79, 179–183. [Google Scholar] [CrossRef]
- Ainsworth, C. 1,2,4-Triazole. Org. Synth. 1960, 40, 99. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, Crysalis-Pro Software System; Version 1.171.38.41; Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Lin, Y.C.; Morris-Natschke, S.L.; Wei, C.F.; Shen, T.C.; Lin, H.Y.; Hsu, M.H.; Chou, L.C.; Zhao, Y.; Kuo, S.C.; et al. Synthesis and SAR studies of novel 6,7,8-substituted 4-substituted benzyloxyquinolin-2(1H)-one derivatives for anticancer activity. Br. J. Pharmacol. 2015, 172, 1195–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| D—H∙∙∙A | D—H | H∙∙∙A | D∙∙∙A | D—H∙∙∙A |
|---|---|---|---|---|
| N8—H8∙∙∙O12 i | 0.85 (2) | 1.95 (2) | 2.7844 (17) | 168 (2) |
| C11—H11B∙∙∙N3 ii | 0.99 | 2.61 | 3.603 (2) | 179 |
| Compound | 60 Cell Lines Assay in One Dose, 10 μM | ||||
|---|---|---|---|---|---|
| Mean Growth, % | Range of Growth, % | Most Sensitive Cell Line(s) (Growth Inhibition Percent)/Panel | Positive Cytostatic Effect a | Positive Cytotoxic Effect b | |
| 2a | 89.98 | 52.79 to 122.91 | NCI-H522 (52.79)/NSCLC# | 0/59 | 0/59 |
| 2c | 102.57 | 77.10 to 146.05 | NCI-H522 (77.10)/NSCLC# | 0/59 | 0/59 |
| 2d | 99.67 | −73.92 to 147.73 | CCRF-CEM (-73.92), HL-60 (TB) (−58.44), MOLT-4 (−73.62) all Leukemia | 2/58 | 3/58 |
| 2e | 101.82 | 61.87 to 133.86 | SR (61.87)/Leukemia | 0/58 | 0/58 |
| 2f | 108.16 | 86.65 to 261.80 | OVCAR-5 (86.65)/Ovarian Cancer | 0/57 | 0/57 |
| 2h | 28.98 | −48.01 to 121.70 | HL-60 (TB) (−32.79), MOLT-4 (−11.15), RPMI-8226 (−16,52), SR (−48.01)/all Leukemia; HOP-92 (−21.66)/NSCLC#; BT-549 (−16.67), HS 578T (−15.94)/all Breast Cancer; RXF 393 (−15.19)/Renal Cancer; LOX IMVI (−9.62)/Melanoma; DU-145 (−21.71)/Prostate Cancer; SF-539 (−33.43), SNB-75 (−11.46)/CNS Cancer | 30/57 | 12/57 |
| 2i | 61.05 | −22.54 to 122.94 | CCRF-CEM (−3.49); SR (−25.54) all Leukemia | 17/58 | 2/58 |
| 2j | 106.32 | 82.41 to 135.61 | K-562 (82.41)/Leukemia | 0/56 | 0/56 |
| 2k | 110.51 | 25.23 to 180.21 | SR (25.23)/Leukemia | 1/56 | 0/56 |
| 2l | 105.41 | 66.88 to 141.12 | PC-3 (66.88)/Prostate Cancer | 0/59 | 0/59 |
| 3 | 97.21 | 74.85 to 122.06 | SR (74.85)/Leukemia | 0/59 | 0/59 |
| 5e | 100.59 | 80.34 to 137.75 | T-47D (80.34)/Breast Cancer | 0/59 | 0/59 |
| 5f | 93.61 | 54.41 to 111.61 | T-47D (54.41)/Breast Cancer | 0/59 | 0/59 |
| Panel, MG_MID | GI50, μM | SI (GI50) | TGI, μM | SI (TGI) | LC50, μM | SI (LC50) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2h | 2i | 2h | 2i | 2h | 2i | 2h | 2i | 2h | 2i | 2h | 2i | |
| Leukemia | 1.92 | 11.32 | 1.84 | 0.97 | 6.85 | 51.55 | 1.88 | 0.55 | 26.81 | 99.25 | 1.39 | 0.64 |
| Range* | 1.35–2.69 | 2.88–18.19 | - | - | 3.16– 14.45 | 9.99– >100 | - | - | 7.58– 48.97 | 95.49– >100 | - | - |
| NSCLC | 6.98 | 14.13 | 0.51 | 0.78 | 18.22 | 30.03 | 0.71 | 0.94 | 44.01 | 64.55 | 0.84 | 0.97 |
| Range* | 2.29–15.48 | 10.23–19.49 | - | - | 9.99– 30.90 | 25.70– 39.81 | - | - | 31.62– 60.25 | 50.11– 83.17 | - | - |
| Colon cancer | 6.13 | 12.42 | 0.58 | 0.88 | 17.39 | 26.51 | 0.74 | 1.06 | 41.39 | 56.32 | 0.90 | 1.12 |
| Range* | 2.45–9.33 | 9.55–16.59 | - | - | 10.96– 21.37 | 21.37– 33.11 | - | - | 33.11– 45.70 | 46.77– 57.60 | - | - |
| CNS Cancer | 2.87 | 9.85 | 1.23 | 1.11 | 10.76 | 27.42 | 1.19 | 1.03 | 38.58 | 66.25 | 0.96 | 0.95 |
| Range* | 1.77–4.07 | 6.02–13.18 | - | - | 4.46– 15.48 | 19.05– 44.66 | - | - | 12.59– 87.09 | 43.65– >100 | - | - |
| Melanoma | 4.79 | 11.77 | 0.74 | 0.93 | 16.49 | 25.61 | 0.78 | 1.10 | 41.20 | 55.37 | 0.90 | 1.14 |
| Range* | 2.63–8.31 | 5.62–14.79 | - | - | 11.22– 20.89 | 18.19– 33.11 | - | - | 33.11– 51.28 | 42.65– 77.62 | - | - |
| Ovarian Cancer | 8.05 | 13.69 | 0.44 | 0.80 | 20.34 | 31.15 | 0.63 | 0.90 | 52.09 | 68.65 | 0.71 | 0.92 |
| Range* | 2.11– 15.48 | 6.60–12.88 | - | - | 5.62– 30.90 | 20.89– 38.90 | - | - | 19.49– 97.72 | 51.28– >100 | - | - |
| Renal Cancer | 5.37 | 13.02 | 0.66 | 0.84 | 16.40 | 27.13 | 0.78 | 1.03 | 40.46 | 55.02 | 0.92 | 1.15 |
| Range* | 1.62– 12.58 | 5.75–17.37 | - | - | 8.12– 25.11 | 21.37– 31.62 | - | - | 28.84– 50.11 | 51.28– 58.88 | - | - |
| Prostate Cancer | 2.67 | 7.6 | 1.33 | 1.44 | 10.34 | 27.11 | 1.25 | 1.04 | 30.77 | 68.15 | 1.21 | 0.93 |
| Range* | 1.54–3.80 | 3.98–11.22 | - | - | 4.46– 16.21 | 16.21– 38.01 | - | - | 15.84– 45.70 | 36.30– >100 | - | - |
| Breast Cancer | 4.48 | 9.72 | 0.79 | 1.13 | 15.61 | 30.29 | 0.82 | 0.93 | 46.05 | 73.18 | 0.80 | 0.86 |
| Range* | <0.01– 15.48 | 3.54–21.87 | - | - | 4.07– 41.68 | 15.84– 67.06 | - | - | 19.49– >100 | 41.68– >100 | - | - |
| Total MG_MID | 3.54 | 10.96 | - | - | 12.88 | 28.18 | - | - | 37.15 | 63.09 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules 2021, 26, 1162. https://doi.org/10.3390/molecules26041162
Holota S, Komykhov S, Sysak S, Gzella A, Cherkas A, Lesyk R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules. 2021; 26(4):1162. https://doi.org/10.3390/molecules26041162
Chicago/Turabian StyleHolota, Serhii, Sergiy Komykhov, Stepan Sysak, Andrzej Gzella, Andriy Cherkas, and Roman Lesyk. 2021. "Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents" Molecules 26, no. 4: 1162. https://doi.org/10.3390/molecules26041162
APA StyleHolota, S., Komykhov, S., Sysak, S., Gzella, A., Cherkas, A., & Lesyk, R. (2021). Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules, 26(4), 1162. https://doi.org/10.3390/molecules26041162