Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics
Abstract
:1. Introduction
2. Results and Discussion
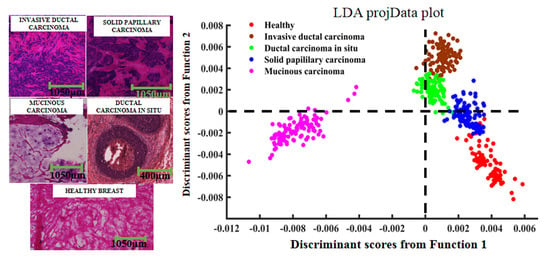
2.1. Pathological Analysis
2.2. Raman Spectral Analysis
2.3. PCA–LDA Analysis
3. Experimental Section
3.1. Sample Preparation
3.2. Spectroscopic Acquisition
3.3. Data Pre-Processing and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowl, J.H.; Stein, K.D.; Alteri, R.; Jemal, D.V.M.A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 252–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koya, K.S.K.; Salutatorian, M.; Yurgelevic, S.; Huang, C.; Werner, C.W.; Kast, R.E.; Shanley, J.; Sherman, M.; Honn, K.V.; Maddipati, K.R.; et al. Accurate identification of breast cancer margins in microenvironments of ex-vivo basal and luminal breast cancer tissues using Raman spectroscopy. Prostag. Other Lipid Mediat. 2020, 151, 106475. [Google Scholar] [CrossRef]
- Sabtu, S.N.; Sani, S.F.; Bradley, D.A.; Looi, L.M.; Osman, Z. A review of the applications of Raman spectroscopy for breast cancer tissue diagnostic and their histopathological classification of epithelial to mesenchymal transition. J. Raman Spectrosc. 2019, 51, 1–10. [Google Scholar]
- Zhao, J.; Zeng, H.; Kalia, S.; Lui, H. Wavenumber selection based analysis in Raman spectroscopy improves skin cancer diagnostic specificity. Analyst 2016, 141, 1034–1043. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-Cancer Screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 373, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.A. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer 2003, 97, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, R.; Cromwell, D.A.; Trivella, M. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ 2012, 345, e4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccahill, L.E.; Single, R.M.; Aiello Bowles, E.J.; Feigelson, H.S.; James, T.A.; Barney, T.; Engel, J.M.; Onitilo, A.A. Variability in Reexcision Following Breast Conservation Surgery. JAMA J. Am. Med. Assoc. 2012, 307, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Keating, J.J.; Fisher, C.; Batiste, R.; Singhal, S. Advances in Intraoperative Margin Assessment for Breast Cancer. Curr. Surg. Rep. 2016, 4, 15. [Google Scholar] [CrossRef]
- Jorns, J.M.; Daignault, S.; Sabel, M.S.; Wu, A.J. Is Intraoperative Frozen Section Analysis of Reexcision Specimens of Value in Preventing Reoperation in Breast-Conserving Therapy? Am.J. Clin. Pathol. 2014, 142, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, E.L.; Warram, J.M.; Bland, K.I.; Zinn, K.R. The status of contemporary image-guided modalities in oncologic surgery. Ann. Surg. 2015, 261, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Valdes, E.K.; Boolbol, S.K.; Ali, I.; Feldman, S.M.; Cohen, J.M. Intraoperative Touch Preparation Cytology for Margin Assessment in Breast-Conservation Surgery: Does It Work for Lobular Carcinoma? Ann. Surg. Oncol. 2007, 14, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- David, I.E.; David, P.C.; Lorna, A.; Steve, O.; Royston, G. Illuminating disease and enlightening biomedicine: Raman spectroscopy as a diagnostic tool. Analyst 2013, 138, 3871–3884. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metast. Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Yu, F.; Chen, S.; Chen, Y.; He, Q.; Zhang, Z.; Zhang, J.; Wang, S. Raman spectroscopy combined with multivariate analysis to study the biochemical mechanism of lung cancer microwave ablation. Biomed. Opt. Express 2020, 11, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Chen, T.; Wang, S.; Chen, S.; Li, H.; Yu, F.; Zhang, J.; Zhang, Z. Study on the biochemical mechanisms of the micro-wave ablation treatment of lung cancer by ex vivo confocal Raman microspectral imaging. Analyst 2020, 145, 626–635. [Google Scholar] [CrossRef]
- Santos, I.P.; Barroso, E.M.; Schut, T.C.B.; Caspers, P.J.; Van Lanschot, C.G.F.; Choi, D.; Der Kamp, M.F.V.; Smits, R.W.H.; Van Doorn, R.; Verdijk, R.M. Raman spectroscopy for cancer detection and cancer surgery guidance: Translation to the clinics. Analyst 2017, 142, 3025–3047. [Google Scholar] [CrossRef]
- Lieber, C.A.; Majumder, S.K.; Billheimer, D.D.; Ellis, L.M.D.D.; Mahadevanjansen, A. Raman microspectroscopy for skin cancer detection in vitro. J. Biomed. Opt. 2008, 13, 024013. [Google Scholar] [CrossRef] [PubMed]
- Vanna, R.; Morasso, C.; Piccotti, F.; Torti, E.; Altamura, D.; Albasini, S.; Agozzino, M.; Villani, L.; Sorrentino, L.; Bunk, O. Raman Spectroscopy Reveals That Biochemical Composition of Breast Microcalcifications Correlates with Histopathologic Features. Cancer Res. 2020, 80, 1762–1772. [Google Scholar] [CrossRef] [Green Version]
- Shipp, D.W.; Rakha, E.A.; Koloydenko, A.A.; Douglas, M.R.; Ellis, I.O.; Ioan, N. Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery. Breast Cancer Res. 2018, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- Garla, V.; Taylor, C.; Brandt, C. Semi-supervised clinical text classification with Laplacian SVMs: An application to cancer case management. J. Biomed. Inform. 2013, 46, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, G.; Kendall, C.; Shepherd, N.A.; Stone, N.J.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Short, M.; Zhao, J.; Wang, H.; Lui, H.; Korbelik, M.; Zeng, H. Full range characterization of the Raman spectra of organs in a murine model. Opt. Express 2011, 19, 22892–22909. [Google Scholar] [CrossRef]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.M.; Rao, L.; Kartha, V.B.; Krishna, C.M. Discrimination of normal, inflammatory, premalignant, and malignant oral tissue: A Raman spectroscopy study. Biopolymers 2010, 81, 179–193. [Google Scholar] [CrossRef]
- Han, B.; Du, Y.; Fu, T.; Fan, Z.; Xu, S.; Hu, C.; Bi, L.; Gao, T.; Zhang, H.; Xu, W. Differences and Relationships Between Normal and Atypical Ductal Hyperplasia, Ductal Carcinoma In Situ, and Invasive Ductal Carcinoma Tissues in the Breast Based on Raman Spectroscopy. Appl. Spectrosc. 2017, 71, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, R.J.; Kartha, V.B.; Krishna, C.M.; Solomon, J.G.R.; Ullas, G.; Devi, P.U. Tissue Raman spectroscopy for the study of radiation damage: Brain irradiation of mice. Radiat. Res. 2002, 157, 175–182. [Google Scholar] [CrossRef]
- Stone, N.J.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef]
- Koljenovic, S.; Schut, T.C.B.; Vincent, A.J.P.E.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef]
- Huang, Z.; Mcwilliams, A.; Lui, H.; Mclean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davis, C.R.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [Green Version]
- Kamemoto, L.E.; Misra, A.K.; Sharma, S.K.; Goodman, M.T.; Acosta, T. Near-Infrared Micro-Raman Spectroscopy for in Vitro Detection of Cervical Cancer. Appl. Spectrosc. 2010, 64, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, T.; Fontana, L.C.; Raniero, L.; Ferreirastrixino, J. In vivo Raman spectroscopy of breast tumors prephotodynamic and postphotodynamic therapy. J. Raman Spectrosc. 2018, 49, 786–791. [Google Scholar] [CrossRef]
- Brozekpluska, B.; Placek, I.; Kurczewski, K.; Morawiec, Z.; Tazbir, M.; Abramczyk, H. Breast cancer diagnostics by Raman spectroscopy. J. Mol. Liq. 2008, 141, 145–148. [Google Scholar] [CrossRef]
- Bonnier, F.; Byrne, H.J. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst 2012, 137, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Chica, A.J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Characterization by Raman spectroscopy of conformational changes on guanine–cytosine and adenine–thymine oligonucleotides induced by aminooxy analogues of spermidine. J. Raman Spectrosc. 2010, 35, 93–100. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Z.; Gong, Y.; Yin, Y.; Wang, K.; He, Q.; Wang, Z.; Bai, J. Confocal raman microspectral imaging of ex vivo human spinal cord tissue. J. Photochem. Photobiol. B Biol. 2016, 163, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Lui, H.; He, Q.; Zeng, H. A modular Raman microspectroscopy system for biological tissue analysis. Spectroscopy 2010, 24, 577–583. [Google Scholar] [CrossRef]
- Shim, M.G.; Wilson, B.C. The effects of ex vivo handling procedures on the near-infrared Raman spectra of normal mammalian tissues. Photochem. Photobiol. 1996, 63, 662–671. [Google Scholar]
- Li, J.; Qin, J.; Zeng, H.; Li, J.; Wang, S. Unveiling doseand time γ: Ependent osteosarcoma cell responses to the γ: Ecretase inhibitor, DAPT, by confocal Raman microscopy. J. Biophoton. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Qin, J.; Zeng, H.; Wang, S. Confocal Raman microspectroscopic analysis on the time-dependent impact of DAPT, a γ-secretase inhibitor, to osteosarcoma cells. Spectrochim. Acta A 2020, 239, 118372. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, R.; Lin, J.; Pan, J.; Chen, G.; Li, Y.; Cheng, M.; Huang, Z.; Chen, J.; Zeng, H. Nasopharyngeal cancer detection based on blood plasma surface-enhanced Raman spectroscopy and multivariate analysis. Biosens. Bioelectron. 2010, 25, 2414–2419. [Google Scholar] [CrossRef] [PubMed]






| Types of Cancer | Number of Samples | Number of Patients |
|---|---|---|
| HC | 12 | 4 |
| SPC | 3 | 3 |
| MC | 3 | 3 |
| IDC | 6 | 6 |
| DCIS | 6 | 6 |
| Actual/Predict | HC | IDC | DCIS | SPC | MC |
|---|---|---|---|---|---|
| HC | 78 | 0 | 0 | 2 | 0 |
| IDC | 0 | 77 | 3 | 0 | 0 |
| DCIS | 0 | 0 | 80 | 0 | 0 |
| SPC | 0 | 0 | 0 | 80 | 0 |
| MC | 0 | 0 | 0 | 0 | 80 |
| Actual/Predict | HC | IDC | DCIS | SPC | MC |
|---|---|---|---|---|---|
| HC | 20 | 0 | 0 | 0 | 0 |
| IDC | 0 | 20 | 0 | 0 | 0 |
| DCIS | 0 | 0 | 20 | 0 | 0 |
| SPC | 0 | 0 | 0 | 20 | 0 |
| MC | 0 | 0 | 0 | 0 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ning, T.; Yu, F.; Chen, Y.; Zhang, B.; Wang, S. Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules 2021, 26, 921. https://doi.org/10.3390/molecules26040921
Li H, Ning T, Yu F, Chen Y, Zhang B, Wang S. Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules. 2021; 26(4):921. https://doi.org/10.3390/molecules26040921
Chicago/Turabian StyleLi, Heping, Tian Ning, Fan Yu, Yishen Chen, Baoping Zhang, and Shuang Wang. 2021. "Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics" Molecules 26, no. 4: 921. https://doi.org/10.3390/molecules26040921
APA StyleLi, H., Ning, T., Yu, F., Chen, Y., Zhang, B., & Wang, S. (2021). Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules, 26(4), 921. https://doi.org/10.3390/molecules26040921