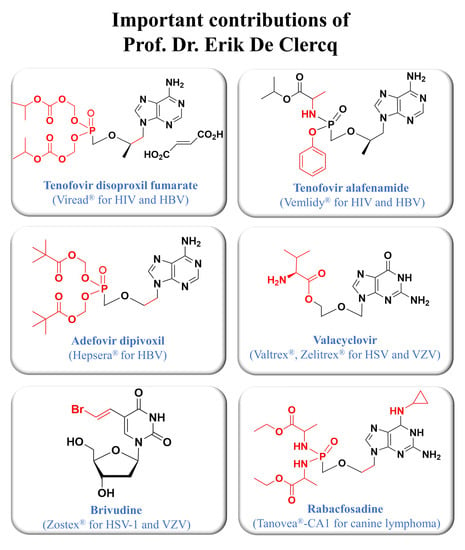
Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday
Abstract
:1. Introduction
2. Adenosine Nucleos(t)ide Analogues
2.1. Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide
- Viread® (tenofovir disoproxil fumarate);
- Truvada® (tenofovir disoproxil fumarate, emtricitabine);
- Atripla® (tenofovir disoproxil fumarate, emtricitabine, efavirenz);
- Eviplera® (tenofovir disoproxil fumarate, emtricitabine, rilpivirine hydrochloride);
- Stribild® (tenofovir disoproxil fumarate, emtricitabine, elvitegravir, cobicistat);
- Symfi® (tenofovir disoproxil fumarate, lamivudine, efavirenz 600 mg);
- Symfi Lo® (tenofovir disoproxil fumarate, lamivudine, efavirenz 400 mg);
- Cimduo® (tenofovir disoproxil fumarate, lamivudine);
- Delstrigo® (tenofovir disoproxil fumarate, lamivudine, doravirine).
- Vemlidy® (tenofovir alafenamide);
- Descovy® (tenofovir alafenamide, emtricitabine);
- Genvoya® (tenofovir alafenamide, emtricitabine, elvitegravir, cobicistat);
- Odefsey® (tenofovir alafenamide, emtricitabine, rilpivirine hydrochloride);
- Biktarvy® (tenofovir alafenamide, emtricitabine, bictegravir);
- Symtuza® (tenofovir alafenamide, emtricitabine, darunavir, cobicistat).
2.2. Adefovir Dipivoxil
2.3. Novel Adenosine Nucleos(t)ide Analogues
3. Thymidine Nucleos(t)ide Analogues
3.1. Brivudine
3.2. Stavudine
4. Guanosine Nucleos(t)ide Analogues
4.1. Valacyclovir
4.2. Rabacfosadine
5. Cytidine Nucleos(t)ide Analogues
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seley-Radtke, K. Discovery, Design, Synthesis, and Application of Nucleoside/Nucleotides. Molecules 2020, 25, 1526. [Google Scholar] [CrossRef] [Green Version]
- Khandazhinskaya, A.L.; Matyugina, E.S.; Solyev, P.N.; Wilkinson, M.; Buckheit, K.W.; Buckheit, R.W., Jr.; Chernousova, L.N.; Smirnova, T.G.; Andreevskaya, S.N.; Alzahrani, K.J.; et al. Investigation of 5′-Norcarbocyclic Nucleoside Analogues as Antiprotozoal and Antibacterial Agents. Molecules 2019, 24, 3433. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [Green Version]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.K.; Chatterjee, P.; Flint, M.; Arefeayne, Y.; Makuc, D.; Plavec, J.; Spiropoulou, C.F.; Seley-Radtke, K.L. Probing the Effects of Pyrimidine Functional Group Switches on Acyclic Fleximer Analogues for Antiviral Activity. Molecules 2019, 24, 3184. [Google Scholar] [CrossRef] [Green Version]
- Venkatesham, A.; Pillalamarri, S.R.; De Wit, F.; Lescrinier, E.; Debyser, Z.; Van Aerschot, A. Propargylated Purine Deoxynucleosides: New Tools for Fluorescence Imaging Strategies. Molecules 2019, 24, 468. [Google Scholar] [CrossRef] [Green Version]
- Dentmon, Z.W.; Kaiser, T.M.; Liotta, D.C. Synthesis and Antiviral Activity of a Series of 2′-C-Methyl-4′-thionucleoside Monophosphate Prodrugs. Molecules 2020, 25, 5165. [Google Scholar] [CrossRef] [PubMed]
- Geant, P.Y.; Uttaro, J.P.; Périgaud, C.; Mathé, C. Synthesis and Antiviral Evaluation of 3′-Fluoro-5′-norcarbocyclic Nucleoside Phosphonates Bearing Uracil and Cytosine as Potential Antiviral Agents. Molecules 2020, 25, 3708. [Google Scholar] [CrossRef]
- Kasthuri, M.; Li, C.; Verma, K.; Russell, O.O.; Dickson, L.; McCormick, L.; Bassit, L.; Amblard, F.; Schinazi, R.F. Synthesis of 4′-Substituted-2′-Deoxy-2′-α-Fluoro Nucleoside Analogs as Potential Antiviral Agents. Molecules 2020, 25, 1258. [Google Scholar] [CrossRef] [Green Version]
- Maslova, A.A.; Matyugina, E.S.; Snoeck, R.; Andrei, G.; Kochetkov, S.N.; Khandazhinskaya, A.L.; Novikov, M.S. Uracil-Containing Heterodimers of a New Type: Synthesis and Study of Their Anti-Viral Properties. Molecules 2020, 25, 3350. [Google Scholar] [CrossRef]
- Bassetto, M.; Cima, C.M.; Basso, M.; Salerno, M.; Schwarze, F.; Friese, D.; Bugert, J.J.; Brancale, A. Novel Nucleoside Analogues as Effective Antiviral Agents for Zika Virus Infections. Molecules 2020, 25, 4813. [Google Scholar] [CrossRef]
- Papadakis, G.; Gerasi, M.; Snoeck, R.; Marakos, P.; Andrei, G.; Lougiakis, N.; Pouli, N. Synthesis of New Imidazopyridine Nucleoside Derivatives Designed as Maribavir Analogues. Molecules 2020, 25, 4531. [Google Scholar] [CrossRef]
- De Clercq, E. C-Nucleosides to Be Revisited. J. Med. Chem. 2016, 59, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Mozobil® (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir. Chem. Chemother. 2019, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. AMD3100/CXCR4 Inhibitor. Front. Immunol. 2015, 6, 276. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; De Clercq, E.; Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020, 16, 11–30. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. The AMD3100 story: The path to the discovery of a stem cell mobilizer (Mozobil). Biochem. Pharmacol. 2009, 77, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. Highlights in the discovery of antiviral drugs: A personal retrospective. J. Med. Chem. 2010, 53, 1438–1450. [Google Scholar] [CrossRef]
- De Clercq, E. Curious discoveries in antiviral drug development: The role of serendipity. Med. Res. Rev. 2015, 35, 698–719. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parang, K.; El-Sayed, N.S.; Kazeminy, A.J.; Tiwari, R.K. Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs Against Human Coronavirus 229E (HCoV-229E). Molecules 2020, 25, 2343. [Google Scholar] [CrossRef]
- Miao, M.; Jing, X.; De Clercq, E.; Li, G. Danoprevir for the Treatment of Hepatitis C Virus Infection: Design, Development, and Place in Therapy. Drug Des. Devel. Ther. 2020, 14, 2759–2774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, G.; Shang, J.; Pan, C.; Zhang, M.; Yin, Z.; Xie, Q.; Peng, Y.; Mao, Q.; Xiao, X.; et al. Rapidly decreased HBV RNA predicts responses of pegylated interferons in HBeAg-positive patients: A longitudinal cohort study. Hepatol. Int. 2020, 14, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Balzarini, J.; Holý, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; De Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Follis, K.E.; Sabo, A.; Beck, T.W.; Grant, R.F.; Bischofberger, N.; Benveniste, R.E.; Black, R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 1995, 270, 1197–1199. [Google Scholar] [CrossRef]
- De Clercq, E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; De Clercq, E.; Li, G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 813–829. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem. Pharmacol. 2018, 153, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Zolopa, A.; Brar, I.; Elion, R.; Ortiz, R.; Post, F.; Wang, H.; Callebaut, C.; Martin, H.; Fordyce, M.W.; et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: A randomized phase 2 study. J. Acquir. Immune Defic. Syndr. 2014, 67, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapia, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Feng, Y.; Yan, X. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J. Hepatol. 2021, 74, 245–246. [Google Scholar] [CrossRef]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.J.; Lim, Y.S.; Mehta, R.; Janssen, H.L.; et al. GS-US-320-0108 Investigators, Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Agarwal, K.; Brunetto, M.; Seto, W.K.; Lim, Y.S.; Fung, S.; Marcellin, P.; Ahn, S.H.; Izumi, N.; Chuang, W.L.; Bae, H.; et al. Investigators, 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 2018, 68, 672–681. [Google Scholar] [CrossRef]
- Cathcart, A.L.; Chan, H.L.; Bhardwaj, N.; Liu, Y.; Marcellin, P.; Pan, C.Q.; Shalimar Buti, M.; Cox, S.; Parhy, B.; Zhou, E.; et al. No Resistance to Tenofovir Alafenamide Detected through 96 Weeks of Treatment in Patients with Chronic Hepatitis B Infection. Antimicrob. Agents Chemother. 2018, 62, e01064–e01118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Fraga, R.S.; Van Vaisberg, V.; Mendes, L.C.A.; Carrilho, F.J.; Ono, S.K. Adverse events of nucleos(t)ide analogues for chronic hepatitis B: A systematic review. J. Gastroenterol. 2020, 55, 496–514. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. The history of antiretrovirals: Key discoveries over the past 25 years. Rev. Med. Virol. 2009, 19, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Wulfsohn, M.S.; et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 2003, 348, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Marcellin, P.; Chang, T.T.; Lim, S.G.; Tong, M.J.; Sievert, W.; Shiffman, M.L.; Jeffers, L.; Goodman, Z.; Wulfsohn, M.S.; Xiong, S.; et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 2003, 348, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Marcellin, P.; Heathcote, E.J.; Buti, M.; Gane, E.; de Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008, 359, 2442–2455. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Higashi-Kuwata, N.; Hayashi, S.; Kumamoto, H.; Ogata-Aoki, H.; Das, D.; Venzon, D.; Hattori, S.I.; Bulut, H.; Hashimoto, M.; Otagiri, M.; et al. Identification of a novel long-acting 4′-modified nucleoside reverse transcriptase inhibitor against HBV. J. Hepatol. 2020. [Google Scholar] [CrossRef]
- Eyer, L.; Svoboda, P.; Balvan, J.; Vičar, T.; Raudenska, M.; Štefánik, M.; Haviernik, J.; Huvarova, I.; Strakova, P.; Rudolf, I.; et al. Broad-Spectrum Antiviral Activity of 3′-Deoxy-3′-Fluoroadenosine against Emerging Flaviviruses. Antimicrob. Agents Chemother. 2021, 65, e01522-20. [Google Scholar]
- Tanase, C.I.; Draghici, C.; Hanganu, A.; Pintilie, L.; Maganu, M.; Volobueva, A.; Sinegubova, E.; Zarubaev, V.V.; Neyts, J.; Jochmans, D.; et al. New HSV-1 Anti-Viral 1′-Homocarbocyclic Nucleoside Analogs with an Optically Active Substituted Bicyclo[2.2.1]Heptane Fragment as a Glycoside Moiety. Molecules 2019, 24, 2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. An Odyssey in antiviral drug development-50 years at the Rega Institute: 1964–2014. Acta Pharm. Sin. B 2015, 5, 520–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E.; Descamps, J.; De Somer, P.; Barr, P.J.; Jones, A.S.; Walker, R.T. (E)-5-(2-Bromovinyl)-2′-deoxyuridine: A potent and selective anti-herpes agent. Proc. Natl. Acad. Sci. USA 1979, 76, 2947–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E.; Degreef, H.; Wildiers, J.; De Jonge, G.; Drochmans, A.; Descamps, J.; De Somer, P. Oral (E)-5-(2-bromovinyl)-2′-deoxyuridine in severe herpes zoster. Br. Med. J. 1980, 281, 1178. [Google Scholar] [CrossRef] [Green Version]
- Andrei, G.; Sienaert, R.; McGuigan, C.; De Clercq, E.; Balzarini, J.; Snoeck, R. Susceptibilities of several clinical varicella-zoster virus (VZV) isolates and drug-resistant VZV strains to bicyclic furano pyrimidine nucleosides. Antimicrob. Agents Chemother. 2005, 49, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, M.; Pauwels, R.; Herdewijn, P.; De Clercq, E.; Desmyter, J.; Vandeputte, M. Both 2′,3′-dideoxythymidine and its 2′,3′-unsaturated derivative (2′,3′-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem. Biophys. Res. Commun. 1987, 142, 128–134. [Google Scholar] [CrossRef]
- Bertoletti, N.; Chan, A.H.; Schinazi, R.F.; Anderson, K.S. Post-Catalytic Complexes with Emtricitabine or Stavudine and HIV-1 Reverse Transcriptase Reveal New Mechanistic Insights for Nucleotide Incorporation and Drug Resistance. Molecules 2020, 25, 4868. [Google Scholar] [CrossRef] [PubMed]
- Colla, L.; De Clercq, E.; Busson, R.; Vanderhaeghe, H. Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine]. J. Med. Chem. 1983, 26, 602–604. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. The discovery of antiviral agents: Ten different compounds, ten different stories. Med. Res. Rev. 2008, 28, 929–953. [Google Scholar] [CrossRef]
- Maudgal, P.C.; De Clercq, K.; Descamps, J.; Missotten, L. Topical treatment of experimental herpes simplex keratouveitis with 2′-O-glycylacyclovir. A water-soluble ester of acyclovir. Arch. Ophthalmol. 1984, 102, 140–142. [Google Scholar] [CrossRef]
- Tyring, S.K.; Baker, D.; Snowden, W. Valacyclovir for herpes simplex virus infection: Long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J. Infect. Dis. 2002, 186 (Suppl. 1), S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Jones, C.; Ville, Y. Congenital cytomegalovirus infection: Management update. Curr. Opin. Infect. Dis. 2017, 30, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Ville, S.; Imbert-Marcille, B.M.; Coste-Burel, M.; Garandeau, C.; Meurette, A.; Cantarovitch, D.; Giral, M.; Hourmant, M.; Blancho, G.; Dantal, J. Impact of antiviral prophylaxis in adults Epstein-Barr Virus-seronegative kidney recipients on early and late post-transplantation lymphoproliferative disorder onset: A retrospective cohort study. Transpl. Int. 2018, 31, 484–494. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E.; Sakuma, T.; Baba, M.; Pauwels, R.; Balzarini, J.; Rosenberg, I.; Holý, A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral. Res. 1987, 8, 261–272. [Google Scholar] [CrossRef]
- Kramata, P.; Downey, K.M.; Paborsky, L.R. Incorporation and excision of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase delta and epsilon in vitro. J. Biol. Chem. 1998, 273, 21966–21971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naesens, L.; Hatse, S.; Segers, C.; Verbeken, E.; De Clercq, E.; Waer, M.; Balzarini, J. 9-(2-phosphonylmethoxyethyl)-N6-cyclopropyl-2,6-diaminopurine: A novel prodrug of 9-(2-phosphonylmethoxyethyl)guanine with improved antitumor efficacy and selectivity in choriocarcinoma-bearing rats. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 1999, 11, 195–203. [Google Scholar]
- De Clercq, E. Tanovea(R) for the treatment of lymphoma in dogs. Biochem. Pharmacol. 2018, 154, 265–269. [Google Scholar] [CrossRef]
- Reiser, H.; Wang, J.; Chong, L.; Watkins, W.J.; Ray, A.S.; Shibata, R.; Birkus, G.; Cihlar, T.; Wu, S.; Li, B.; et al. GS-9219—A novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin’s lymphoma. Clin. Cancer Res. 2008, 14, 2824–2832. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. The clinical potential of the acyclic (and cyclic) nucleoside phosphonates: The magic of the phosphonate bond. Biochem. Pharmacol. 2011, 82, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. Where rilpivirine meets with tenofovir, the start of a new anti-HIV drug combination era. Biochem. Pharmacol. 2012, 84, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Trompet, E.; Snoeck, R. Novel Therapeutics for Epstein—Barr Virus. Molecules 2019, 24, 997. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, T.; Wakisaka, N.; Kondo, S.; Murono, S.; Shimizu, Y.; Nakashima, M.; Tsuji, A.; Furukawa, M. Treatment of locally recurrent Epstein-Barr virus-associated nasopharyngeal carcinoma using the anti-viral agent cidofovir. J. Med. Virol. 2008, 80, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malin, J.J.; Suárez, I.; Priesner, V.; Fatkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin. Microbiol. Rev. 2021, 34, e00162-20. [Google Scholar]
- Zhou, Z.; Zhang, M.; Wang, Y.; Zheng, F.; Huang, Y.; Huang, K.; Yu, Q.; Cai, C.; Chen, D.; Tian, Y.; et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging 2020, 12, 11296–11305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Transl. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, Y.; Jing, X.; Wang, Y.; Miao, M.; Tao, L.; Zhou, Z.; Xie, Y.; Huang, Y.; Lei, J.; et al. Mortality risk of COVID-19 in elderly males with comorbidities: A multi-country study. Aging 2020, 13, 27–60. [Google Scholar]
- Yousefi, H.; Mashouri, L.; Okpechi, S.C.; Alahari, N.; Alahari, S.K. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: A review describing drug mechanisms of action. Biochem. Pharmacol. 2021, 183, 114296. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Y. Addressing the selectivity and toxicity of antiviral nucleosides. Antivir. Chem. Chemother. 2018, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]





| Drug Name * | Brand Name | Diseases | Clinical Use in Adults | Agency ‡ | Approval Date |
|---|---|---|---|---|---|
| AdenosineNucleotideAnalogues | |||||
| Tenofovir disoproxil fumarate | Viread® | HIV, HBV | One 300 mg tablet/day taken orally | U.S. FDA | 2001 |
| EMA | 2002 | ||||
| Tenofovir alafenamide | Vemlidy® | HIV, HBV | One 25 mg tablet/day taken with food | U.S. FDA | 2015 |
| EMA | 2017 | ||||
| Adefovir dipivoxil | Hepsera® | HBV | One 10 mg tablet/day taken orally with or without food | U.S. FDA | 2002 |
| EMA | 2003 | ||||
| Thymidine Nucleoside Analogues | |||||
| Stavudine | Zerit® | HIV | Adults < 60 kg: 30 mg/per 12 h | U.S. FDA | 1994 |
| Adults ≥ 60 kg: 40 mg/per 12 h | EMA | 1996 | |||
| Brivudine | Zostex® and others | HSV-1, VZV | One 125 mg tablet/day orally for 7 days | ‡ | 2000 |
| Guanosine Analogues | |||||
| Valacyclovir | Valtrex®, Zelitrex® | HSV | Cold sores: 2 g/12 h for 1 day | U.S. FDA | 1995 |
| Genital herpes: ≥ 500 mg/day # | EMA | 2010 | |||
| VZV | 1 g three times daily for 7 days | ||||
| Rabacfosadine (GS-9219) | Tanovea®-CA1 | Lymphoma in dogs | 1 mg/kg as a 30-min intravenous infusion, once every 3 weeks, for up to five doses | U.S. FDA | 2016 |
| Cytidine Nucleotide Analogue | |||||
| Cidofovir | Vistide® | HCMV retinitis (AIDS patients) | Intravenous infusion of 5 mg/kg dose administered biweekly | U.S. FDA | 1996 |
| EMA | 1997 | ||||
| Bicyclam Derivative | |||||
| Plerixafor (AMD3100) | Mozobil® | Multiple myeloma, non-Hodgkin’s lymphoma | Initiate Mozobil treatment after G-CSF per day for 4 days. Subcutaneous injection of weight-adjusted Mozobil dose approximately 11 h before the initiation of apheresis up to 4 consecutive days | U.S. FDA | 2008 |
| EMA | 2009 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yue, T.; Zhang, P.; Gu, W.; Gao, L.-J.; Tan, L. Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules 2021, 26, 923. https://doi.org/10.3390/molecules26040923
Li G, Yue T, Zhang P, Gu W, Gao L-J, Tan L. Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules. 2021; 26(4):923. https://doi.org/10.3390/molecules26040923
Chicago/Turabian StyleLi, Guangdi, Tingting Yue, Pan Zhang, Weijie Gu, Ling-Jie Gao, and Li Tan. 2021. "Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday" Molecules 26, no. 4: 923. https://doi.org/10.3390/molecules26040923
APA StyleLi, G., Yue, T., Zhang, P., Gu, W., Gao, L. -J., & Tan, L. (2021). Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules, 26(4), 923. https://doi.org/10.3390/molecules26040923