Cyanogenic Glycoside Analysis in American Elderberry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Picrate Paper Method
2.2. UHPLC MS/MS Method of Analysis
2.2.1. Method Development and Optimization
2.2.2. Optimized Extraction, Recovery and Matrix Effect
2.2.3. Optimized SPE Method
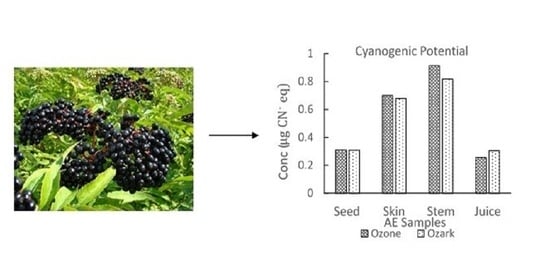
2.2.4. Sample Test
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. American Elderberry Samples
3.3. Sample Preparation and Extraction
3.4. Solid Phase Extraction (SPE)
3.5. Picrate Paper Method of Analysis
3.6. UHPLC-MS/MS Method of Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ACN | acetonitrile |
| AE | American elderberry |
| APCI | atmospheric pressure chemical ionization |
| CDC | Centers for Disease Control and Prevention |
| CNGs | cyanogenic glycosides |
| CNS | cyanogenic standards |
| EE | European elderberry |
| ESI | electrospray ionization |
| FA | formic acid |
| HCN | hydrogen cyanide |
| HPLC-DAD | High-performance liquid chromatography with photo diode array detectors |
| LLOQ | lower limit of quantification |
| LLOD | lower limit of detection |
| ME | matrix effect |
| MRM | multiple reaction monitoring |
| RE | recovery |
| RT | retention time |
| SD | standard deviation |
| SIR | selected ion recording |
| S/N | signal to noise ration |
| SPE | solid-phase extraction |
| TCP | total cyanogenic potential |
| UHPLC-QqQ-MS/MS | Ultra-high performance liquid chromatography triple-quadrupole mass spectrometry |
| ULOQ | upper limit of quantification |
| UV-Vis | ultraviolet visible spectrophotometry |
References
- Byers, P.L.; Thomas, A.L.; Cernusca, M.M.; Godsey, L.D.; Gold, M.A. Growing and Marketing Elderberries; in Missouri. Agroforestry in Action Pub, AF1016; Univ. Missouri Center for Agroforestry: Columbia, MO, USA, 2014. [Google Scholar]
- Thomas, A.L.; Byers, P.L.; Avery, J.D., Jr.; Kaps, M.; Gu, S. Horticultural Performance of Eight American Elderberry Genotypes at Three Missouri Locations. Acta Hortic. 2015, 1061, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [Green Version]
- Moerman, D.E. Native American Ethnobotany; Timber Press: Portland, OR, USA, 2002. [Google Scholar]
- Thomas, A.L.; Byers, P.L.; Vincent, P.L.; Applequist, W.L. Medicinal Attributes of American Elderberry. In Medicinal and Aromatic Plants of North America; Máthé, Á., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 119–139. [Google Scholar]
- Elderberry: Plant Profile. Available online: http://stitchandboots.com/kitchen-garden/fruit/elderberry-plant-profile/ (accessed on 22 December 2020).
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Barak, V.; Birkenfeld, S.; Halperin, T.; Kalickman, I. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Isr. Med. Assoc. J. 2002, 4, 919–922. [Google Scholar]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Uncini Manganelli, R.E.; Zaccaro, L.; Tomei, P.E. Antiviral activity in vitro of Urtica dioica L., Parietaria diffusa M. et K. and Sambucus nigra L. J. Ethnopharmacol. 2005, 98, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Johnson, M.C.; Lu, C.H.; Fritsche, K.L.; Thomas, A.L.; Cai, Z.; Greenlief, C.M. Determination of Anthocyanins and Total Polyphenols in a Variety of Elderberry Juices by UPLC-MS/MS and Other Methods. Acta Hortic. 2015, 1061, 43–51. [Google Scholar] [CrossRef]
- Mohammadsadeghi, S.; Malekpour, A.; Zahedi, S.; Eskandari, F. The Antimicrobial Activity of Elderberry (Sambucus nigra L.) Extract Against Gram Positive Bacteria, Gram Negative Bacteria and Yeast. Res. J. Appl. Sci. 2013, 8, 240–243. [Google Scholar]
- Antolak, H.; Czyzowska, A.; Kregiel, D. Antibacterial and Antiadhesive Activities of Extracts from Edible Plants against Soft Drink Spoilage by Asaia spp. J. Food Prot. 2017, 80, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Werlein, H.D.; Kütemeyer, C.; Schatton, G.; Hubbermann, E.M.; Schwarz, K. Influence of elderberry and blackcurrant concentrates on the growth of microorganisms. Food Control 2005, 16, 729–733. [Google Scholar] [CrossRef]
- Mohebalian, P.M.; Aguilar, F.X.; Cernusca, M.M. Conjoint Analysis of U.S. Consumers’ Preference for Elderberry Jelly and Juice Products. HortScience 2013, 48, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; May, G.; Eckl, V.; Reynolds, C.M. US Sales of Herbal Supplements Increase by 8.6% in 2019. HerbalGram 2020, 127, 54–69. [Google Scholar]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, G.; Cottrell, T. Carotenoids and cyanogenic glucosides in saskatoon berries (Amelanchier alnifolia Nutt). J. Food Compos. Anal. 2008, 21, 249–254. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Buhrmester, R.A.; Ebinger, J.E.; Seigler, D.S. Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. (Caprifoliaceae). Biochem. Syst. Ecol. 2000, 28, 689–695. [Google Scholar] [CrossRef]
- Ganjewala, D. Advances in cyanogenic glycosides biosynthesis and analyses in plants: A review. Acta Biol. Szeged. 2010, 54, 1–14. [Google Scholar]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Abraham, K.; Buhrke, T.; Lampen, A. Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: A crossover study in humans. Arch. Toxicol. 2016, 90, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Zhang, G.; Wood, E.; Rogel Castillo, C.; Mitchell, A.E. Quantification of amygdalin in nonbitter, semibitter, and bitter almonds (Prunus dulcis) by UHPLC-(ESI)QqQ MS/MS. J. Agric. Food Chem. 2013, 61, 7754–7759. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, R.; Howad, W.; Garcia-Mas, J.; Arús, P.; Martínez-Gómez, P.; Dicenta, F. Molecular markers for kernel bitterness in almond. Tree Genet. Genomes 2010, 6, 237–245. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Rasmussen, A.V.; Jorgensen, B.; Naumann, C.M.; Lindberg Moller, B. Cyanogenic glucosides and plant-insect interactions. Phytochemistry 2004, 65, 293–306. [Google Scholar] [CrossRef]
- PDQ® Integrative, A., and Complementary Therapies Editorial Board PDQ Laetrile/Amygdalin. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/laetrile-pdq (accessed on 22 December 2020).
- Sarker, S.D.; Nahar, L. Chemistry for Pharmacy Students General, Organic, and Natural Produce Chemistry; John Wiley and Sons: Chichester, UK, 2007. [Google Scholar]
- Burns, A.E.; Bradbury, J.H.; Cavagnaro, T.R.; Gleadow, R.M. Total cyanide content of cassava food products in Australia. J. Food Compos. Anal. 2012, 25, 79–82. [Google Scholar] [CrossRef]
- Geller, R.J.; Barthold, C.; Saiers, J.A.; Hall, A.H. Pediatric cyanide poisoning: Causes, manifestations, management, and unmet needs. Pediatrics 2006, 118, 2146–2158. [Google Scholar] [CrossRef]
- Sahin, S. Cyanide Poisoning in a Children Caused by Apricot Seeds. J. Health Med. Informat. 2011, 2, 1–2. [Google Scholar]
- Sanchez-Verlaan, P.; Geeraerts, T.; Buys, S.; Riu-Poulenc, B.; Cabot, C.; Fourcade, O.; Megarbane, B.; Genestal, M. An unusual cause of severe lactic acidosis: Cyanide poisoning after bitter almond ingestion. Intensive Care Med. 2011, 37, 168–169. [Google Scholar] [CrossRef]
- Akintonwa, A.; Tunwashe, O.L. Fatal cyanide poisoning from Cassava-based meal. Hum. Exp. Toxicol. 1992, 11, 47–49. [Google Scholar] [CrossRef]
- MMWRCDC. Poisoning from Elderberry Juice-California. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00000311.htm (accessed on 22 December 2020).
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Lewandowska, A.; Pilipczuk, T.; Kusznierewicz, B.; Bartoszek, A. Composition of bioactive secondary metabolites and mutagenicity of Sambucus nigra L. Fruit at different stages of ripeness. Acta Alimentaria 2016, 45, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Gleadow, R.M.; Moldrup, M.E.; O’Donnell, N.H.; Stuart, P.N. Drying and processing protocols affect the quantification of cyanogenic glucosides in forage sorghum. J. Sci Food Agric. 2012, 92, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Processing Techniques to Reduce Toxicity and Antinutrients of Cassava for Use as a Staple Food. Comp. Rev. Food Sci. Food Saf. 2009, 8, 17–27. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Akande, K.E.; Fabiyi, E.F. Effect of Processing Methods on Some Antinutritional Factors in Legume Seeds for Poultry Feeding. Int. J. Poult. Sci. 2010, 9, 996–1001. [Google Scholar] [CrossRef]
- Brinker, A.M.; Seigeler, D.S. Determination of cyanide and cyanogenic glycosides from plants. In Plant Toxin Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1992; Volume 13, pp. 359–381. [Google Scholar]
- Analyzing Food for Nutrition Labeling and Hazardous Contaminants, 1st ed.; Jeon, I.; Ikins, W.G. (Eds.) CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Poulton, J.E. Cyanogenic compounds in plants and their toxic effects. In Handbook of Natural Toxins; Keeler, R.F., Tu, W.T., Eds.; Marcel Dekker: New York, NY, USA, 1983; Volume 1, pp. 117–160. [Google Scholar]
- Thomas, A.L.; Byers, P.L.; Gu, S.; Avery, J.D., Jr.; Kaps, M.; Datta, A.; Fernando, L.; Grossi, P.; Rottinghaus, G.E. Occurrence of Polyphenols, Organic Acids, and Sugars among Diverse Elderberry Genotypes Grown in Three Missouri (USA) Locations. Acta Hortic. 2015, 1061, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Douchioiou, G.; Pui, A.; Danac, R.; Basa, C.; Murariu, M. Improved Spectrophotometric Assay of Cyanide with Picric Acid. Rev. Roum. Chim. 2003, 48, 601–606. [Google Scholar]
- Bradbury, M.G.; Egan, S.V.; Bradbury, J.H. Picrate paper kits for determination of total cyanogens in Cassava roots and all forms of cyanogens in Cassava products. J. Sci. Food Agric. 1999, 79, 593–601. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Elsevier Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Drochioiu, G.; Arsene, C.; Murariu, M.; Oniscu, C. Analysis of cyanogens with resorcinol and picrate. Food Chem. Toxicol. 2008, 46, 3540–3545. [Google Scholar] [CrossRef]
- Miller, R.E.; Tuck, K.L. Reports on the distribution of aromatic cyanogenic glycosides in Australian tropical rainforest tree species of the Lauraceae and Sapindaceae. Phytochemistry 2013, 92, 146–152. [Google Scholar] [CrossRef]
- Gonzalez, O.; Blanco, M.E.; Iriarte, G.; Bartolome, L.; Maguregui, M.I.; Alonso, R.M. Bioanalytical chromatographic method validation according to current regulations, with a special focus on the non-well defined parameters limit of quantification, robustness and matrix effect. J. Chromatogr. A 2014, 1353, 10–27. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Borron, S.W. Recognition and treatment of acute cyanide poisoning. J. Emerg. Nurs. 2006, 32 (Suppl. 4), S12–S18. [Google Scholar] [CrossRef]











| Method/µg CN− eq. | ULOQ | LLOQ | R2 |
|---|---|---|---|
| UV-Vis | 50 | 0.14 | 0.9971 |
| Camera-phone | 50 | 1.59 | 0.9889 |
| Parameters ng/mL | MRM Amygdalin | SIR | |||
|---|---|---|---|---|---|
| Amygdalin | Dhurrin | Prunasin | Linamarin | ||
| LLOD | 0.3 | 3 | 3 | 3 | 1 |
| LLOQ | 1 | 10 | 10 | 5 | 5 |
| ULOQ | 8000 | 8000 | 6000 | 6000 | 2000 |
| R2 | 0.9998 | 0.9998 | 0.9983 | 0.9984 | 0.9910 |
| CNG Standards/ | Conc. | MRM | SIR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Recovery% | (ng/mL) | Amygdalin | Amygdalin | Dhurrin | Prunasin | Linamarin | |||||
| Mean RE% | SD% | Mean RE% | SD% | Mean RE% | SD% | Mean RE% | SD% | Mean RE% | SD% | ||
| Sonication | 1000 | 85.40 | 1.01 | 86.14 | 1.61 | 95.34 | 2.1 | 91.35 | 0.95 | 95.26 | 1.98 |
| (30 min at 30 °C) | 100 | 91.43 | 0.92 | 92.14 | 3.11 | 88.54 | 2.04 | 79.14 | 5.1 | 92.1 | 0.96 |
| Overnight shaking | 1000 | 93.69 | 0.31 | 91.40 | 0.98 | 98.19 | 0.73 | 106.98 | 0.86 | 112.21 | 0.62 |
| (16–24 h) | 100 | 81.70 | 3.61 | 101.54 | 2.98 | 109.99 | 2.75 | 87.94 | 1.85 | 98.11 | 2.13 |
| Elderberry Samples | Concentration ± Standard Deviation (µg/g) | ||||
|---|---|---|---|---|---|
| Amygdalin | Dhurrin | Prunasin | Linamarin | ||
| Seeds | Ozone | 2.38 ± 0.09 | 0.27 ± 0.05 | 0.58 ± 0.04 | 0.12 ± 0.06 |
| Ozark | 0.68 ± 0.12 | 0.22 ± 0.03 | 0.36 ± 0.05 | 0.13 ± 0.05 | |
| Juice | Ozone | 1.57 ± 0.08 | 0.70 ± 0.12 | 1.45 ± 0.06 | 0.29 ± 0.03 |
| Ozark | 0.36 ± 0.03 | 0.63 ± 0.04 | 2.36 ± 0.08 | 0.31 ± 0.01 | |
| Skin | Ozone | 6.38 ± 0.40 | 0.12 ± 0.08 | 2.39 ± 0.04 | 0.75 ± 0.06 |
| Ozark | 3.48 ± 0.14 | 1.46 ± 0.20 | 2.53 ± 0.08 | 0.90 ± 0.11 | |
| Stem | Ozone | 5.42 ± 0.12 | 0.94 ± 0.06 | 2.84 ± 0.02 | 0.48 ± 0.04 |
| Ozark | 2.15 ± 0.17 | 1.91 ± 0.03 | 3.07 ± 0.06 | 0.57 ± 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appenteng, M.K.; Krueger, R.; Johnson, M.C.; Ingold, H.; Bell, R.; Thomas, A.L.; Greenlief, C.M. Cyanogenic Glycoside Analysis in American Elderberry. Molecules 2021, 26, 1384. https://doi.org/10.3390/molecules26051384
Appenteng MK, Krueger R, Johnson MC, Ingold H, Bell R, Thomas AL, Greenlief CM. Cyanogenic Glycoside Analysis in American Elderberry. Molecules. 2021; 26(5):1384. https://doi.org/10.3390/molecules26051384
Chicago/Turabian StyleAppenteng, Michael K., Ritter Krueger, Mitch C. Johnson, Harrison Ingold, Richard Bell, Andrew L. Thomas, and C. Michael Greenlief. 2021. "Cyanogenic Glycoside Analysis in American Elderberry" Molecules 26, no. 5: 1384. https://doi.org/10.3390/molecules26051384
APA StyleAppenteng, M. K., Krueger, R., Johnson, M. C., Ingold, H., Bell, R., Thomas, A. L., & Greenlief, C. M. (2021). Cyanogenic Glycoside Analysis in American Elderberry. Molecules, 26(5), 1384. https://doi.org/10.3390/molecules26051384