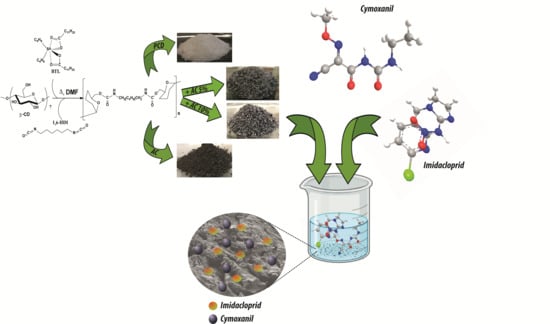
Poly(β-cyclodextrin)-Activated Carbon Gel Composites for Removal of Pesticides from Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Poly(β-cyclodextrin) Synthesis and Characterization
2.2. Sorption Kinetics
2.3. Sorption Isotherms
2.4. Effect of Additives on the Pesticide Sorption Isotherms
2.5. Reusability Assessment
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Poly(β-cyclodextrin) and Its Composites
3.3. Physico–Chemical Characterization of Absorbent Materials
3.4. Sorption Analysis
3.5. Reusability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Paciência, I.; Madureira, J.; Rufo, J.; Moreira, A.; Fernandes, E.D.O. A systematic review of evidence and implications of spatial and seasonal variations of volatile organic compounds (VOC) in indoor human environments. J. Toxicol. Environ. Health Part B 2016, 19, 47–64. [Google Scholar] [CrossRef]
- Mahinroosta, R.; Senevirathna, L. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Pariatamby, A.; Kee, Y.L. Persistent Organic Pollutants Management and Remediation. Procedia Environ. Sci. 2016, 31, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Parween, T.; Jan, S. Pesticide consumption and threats to biodiversity. In Ecophysiology of Pesticides; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–73. ISBN 9780128176146. [Google Scholar]
- Kaur, H.; Garg, H. Pesticides: Environmental Impacts and Management Strategies. In Pesticides—Toxic Aspects; Larramendy, L.M., Soloneski, S., Eds.; InTech: London, UK, 2014; Volume 395, pp. 116–124. [Google Scholar]
- Quarcoo, F.; Bonsi, C.; Tackie, N. Pesticides, the Environment, and Human Health. In Pesticides–Toxic Aspects; Larramendy, L.M., Soloneski, S., Eds.; InTech: London, UK, 2014; p. 13. [Google Scholar]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. Excli J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2018, 25, 2051–2064. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [Green Version]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Shen, T.; Wang, Q.; Wang, X.; Xu, P.; Zheng, Q.; Zhang, G. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review. Sci. Total Environ. 2020, 722, 137831. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Mtunzi, F.M.; Adebowale, K.O. Clay-carbonaceous material composites: Towards a new class of functional adsorbents for water treatment. Surf. Interfaces 2020, 19, 100506. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filho, C.M.C.; Matias, T.; Durães, L.; Valente, A.J.M. Efficient simultaneous removal of petroleum hydrocarbon pollutants by a hydrophobic silica aerogel-like material. Colloids Surf. A Phys. Eng. Asp. 2017, 520, 550–560. [Google Scholar] [CrossRef]
- Addorisio, V.; Pirozzi, D.; Esposito, S.; Sannino, F. Decontamination of waters polluted with simazine by sorption on mesoporous metal oxides. J. Hazard. Mater. 2011, 196, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Reeve, P.J.; Fallowfield, H.J. Natural and surfactant modified zeolites: A review of their applications for water remediation with a focus on surfactant desorption and toxicity towards microorganisms. J. Environ. Manag. 2018, 205, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Hashemi, M.S.; Eslami, F.; Karimzadeh, R. Organic contaminants removal from industrial wastewater by CTAB treated synthetic zeolite Y. J. Environ. Manag. 2019, 233, 785–792. [Google Scholar] [CrossRef]
- Marocco, A.; Dell’Agli, G.; Sannino, F.; Esposito, S.; Bonelli, B.; Allia, P.; Tiberto, P.; Barrera, G.; Pansini, M. Removal of Agrochemicals from Waters by Adsorption: A Critical Comparison among Humic-Like Substances, Zeolites, Porous Oxides, and Magnetic Nanocomposites. Processes 2020, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Suo, F.; Liu, X.; Li, C.; Yuan, M.; Zhang, B.; Wang, J.; Ma, Y.; Lai, Z.; Ji, M. Mesoporous activated carbon from starch for superior rapid pesticides removal. Int. J. Biol. Macromol. 2019, 121, 806–813. [Google Scholar] [CrossRef]
- Bojic, D.; Kostic, M.; Radovic-Vucic, M.; Velinov, N.; Najdanovic, S.; Petrovic, M.; Bojic, A. Removal of the herbicide 2,4-dichlorophenoxyacetic acid from water by using an ultrahighly efficient thermochemically activated carbon. Hem. Ind. 2019, 73, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef]
- Gimeno, O.; Plucinski, P.; Kolaczkowski, S.T.; Rivas, F.J.; Alvarez, P.M. Removal of the Herbicide MCPA by Commercial Activated Carbons: Equilibrium, Kinetics, and Reversibility. Ind. Eng. Chem. Res. 2003, 42, 1076–1086. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Ahmad, K.S. Sorption and Juglans regia-derived activated carbon-mediated removal of aniline-based herbicide Alachlor from contaminated soils. Environ. Earth Sci. 2018, 77, 437. [Google Scholar] [CrossRef]
- Ayranci, E.; Hoda, N. Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef]
- Amézquita-Marroquín, C.P.; Torres-Lozada, P.; Giraldo, L.; Húmpola, P.D.; Rivero, E.; Poon, P.S.; Matos, J.; Moreno-Piraján, J.C. Sustainable production of nanoporous carbons: Kinetics and equilibrium studies in the removal of atrazine. J. Colloid Interface Sci. 2020, 562, 252–267. [Google Scholar] [CrossRef]
- Faur, C.; Métivier-Pignon, H.; Le Cloirec, P. Multicomponent Adsorption of Pesticides onto Activated Carbon Fibers. Adsorption 2005, 11, 479–490. [Google Scholar] [CrossRef]
- Spaltro, A.; Simonetti, S.; Torrellas, S.A.; Rodriguez, J.G.; Ruiz, D.; Juan, A.; Allegretti, P. Adsorption of bentazon on CAT and CARBOPAL activated carbon: Experimental and computational study. Appl. Surf. Sci. 2018, 433, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Njoku, V.O.; Islam, M.A.; Asif, M.; Hameed, B.H. Utilization of sky fruit husk agricultural waste to produce high quality activated carbon for the herbicide bentazon adsorption. Chem. Eng. J. 2014, 251, 183–191. [Google Scholar] [CrossRef]
- Ahmad, K.S. Arachis hypogaea derived activated carbon steered remediation of Benzimidazole based fungicide adsorbed soils. Chem. Ecol. 2019, 35, 576–591. [Google Scholar] [CrossRef]
- Salman, J.M.; Hameed, B.H. Removal of insecticide carbofuran from aqueous solutions by banana stalks activated carbon. J. Hazard. Mater. 2010, 176, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Hosseini, H.; Alahabadi, A. The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chem. Eng. J. 2013, 214, 172–179. [Google Scholar] [CrossRef]
- Ahmad, K.S. Adsorption removal of endosulfan through Saccharum officinarum derived activated carbon from selected soils. J. Cent. South Univ. 2019, 26, 146–157. [Google Scholar] [CrossRef]
- Ahmad, K.S. Exploring the potential of Juglans regia-derived activated carbon for the removal of adsorbed fungicide Ethaboxam from soils. Environ. Monit. Assess. 2018, 190, 737. [Google Scholar] [CrossRef] [PubMed]
- Santana, G.M.; Lelis, R.C.C.; Jaguaribe, E.F.; Morais, R.d.M.; Paes, J.B.; Trugilho, P.F. Development of activated carbon from bamboo (bambusa vulgaris) for pesticide removal from aqueous solutions. Cerne 2017, 23, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.G.; El-Sayed, M.M.H. Removal of imidacloprid pesticide using nanoporous activated carbons produced via pyrolysis of peach stone agricultural wastes. Chem. Eng. Commun. 2020, 1–12. [Google Scholar] [CrossRef]
- Urbain, K.Y.; Fodjo, E.K.; Ardjouma, D.; Serge, B.Y.; Aimé, E.S.; Irié Marc, G.B.; Albert, T. Removal of imidacloprid using activated carbon produced from ricinodendron heudelotii shells. Bull. Chem. Soc. Ethiop. 2018, 31, 397. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.M.R.; Abolghasemi, S.S.; Ashournia, M.; Modaberi, H. Removal of hinosan from underground water using NH4Cl-modified activated carbon from rice husk. Environ. Sci. Pollut. Res. 2019, 26, 20344–20351. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res. 2011, 45, 4047–4055. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Alves, D.C.S.; Gonçalves, J.O.; Coseglio, B.B.; Burgo, T.A.L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S. Adsorption of phenol onto chitosan hydrogel scaffold modified with carbon nanotubes. J. Environ. Chem. Eng. 2019, 7, 103460. [Google Scholar] [CrossRef]
- Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Rubira, A.F.; Muniz, E.C.; Durães, L.; Murtinho, D.M.B.; Valente, A.J.M. Synthesis, characterization and sorption studies of aromatic compounds by hydrogels of chitosan blended with β-cyclodextrin- and PVA-functionalized pectin. Rsc Adv. 2018, 8, 14609–14622. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Chaudhary, J.; Kumar, V.; Thakur, V.K. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Purdy, W.C. Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 1992, 92, 1457–1470. [Google Scholar] [CrossRef]
- Cova, T.F.G.G.; Cruz, S.M.A.; Valente, A.J.M.; Abreu, P.E.; Marques, J.M.C.; Pais, A.A.C.C. Aggregation of Cyclodextrins: Fundamental Issues and Applications. In Cyclodextrin—A Versatile Ingredient; InTech: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Cova, T.F.G.G.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin-based Materials for Removing Micropollutants From Wastewater. Curr. Org. Chem. 2018, 22, 2150–2181. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Cyclodextrin-based adsorbents for the removal of pollutants from wastewater: A review. Environ. Sci. Pollut. Res. 2021, 28, 1317–1340. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Barkács, K.; Gruiz, K.; Varga, E.; Kenyeres, I.; Záray, G.; Szente, L. Removal of hazardous micropollutants from treated wastewater using cyclodextrin bead polymer—A pilot demonstration case. J. Hazard. Mater. 2020, 383, 121181. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Poly-cyclodextrin cryogels with aligned porous structure for removal of polycyclic aromatic hydrocarbons (PAHs) from water. J. Hazard. Mater. 2017, 335, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Saladino, M.L.; Chillura Martino, D.; Lo Meo, P.; Noto, R. Polyaminocyclodextrin nanosponges: Synthesis, characterization and pH-responsive sequestration abilities. Rsc Adv. 2016, 6, 49941–49953. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and characterization of novel β-cyclodextrin based copolymer materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Silva, D.C.; Jesus, S.; Carvalho, R.A.; Rubira, A.F.; Muniz, E.C.; Borges, O.; Valente, A.J.M. Synthesis and controlled curcumin supramolecular complex release from pH-sensitive modified gum-arabic-based hydrogels. RSC Adv. 2015, 5, 94519–94533. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Anne, J.M.; Boon, Y.H.; Saad, B.; Miskam, M.; Yusoff, M.M.; Shahriman, M.S.; Zain, N.N.M.; Lim, V.; Raoov, M. β-Cyclodextrin conjugated bifunctional isocyanate linker polymer for enhanced removal of 2,4-dinitrophenol from environmental waters. R. Soc. Open Sci. 2018, 5, 180942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Q.; Qi, F.; Cheng, G.; Zhang, Y.; Xiao, B.; Hu, Z.; Liu, S.; Cai, H.; Xu, S. Thermogravimetric analysis of co-combustion of biomass and biochar. J. Therm. Anal. Calorim. 2013, 112, 1475–1479. [Google Scholar] [CrossRef]
- Jin, Z. (Ed.) Cyclodextrin Chemistry; World Scientific Publishing: Singapore, 2013; ISBN 978-981-4436-79-3. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Infrared Spectrometry. In Spectrometric Identification of Organic Compounds; Brennan, D., Yee, J., Wolfman-Robichaud, S., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2005; pp. 72–126. ISBN 0-471-39362-2. [Google Scholar]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative Sorption of Pb and Cd by Biochars and Its Implication for Metal Immobilization in Soils. WaterAirSoil Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M.; Ammar, N. Preparation, modification and industrial application of activated carbon from almond shell. J. Ind. Eng. Chem. 2013, 19, 2092–2099. [Google Scholar] [CrossRef]
- Mallard, I.; Baudelet, D.; Castiglione, F.; Ferro, M.; Panzeri, W.; Ragg, E.; Mele, A. Polydisperse methyl β-cyclodextrin–epichlorohydrin polymers: Variable contact time 13 C CP-MAS solid-state NMR characterization. Beilstein J. Org. Chem. 2015, 11, 2785–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Naghash, H.J.; Okay, O. Formation and structure of polyacrylamide gels. J. Appl. Polym. Sci. 1996, 60, 971–979. [Google Scholar] [CrossRef]
- Estrada, F.G.A.; Marques, J.M.C.; Valente, A.J.M. Molecular Dynamics Insights for Screening the Ability of Polymers to Remove Pesticides from Water. ChemistryOpen 2019, 8, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Spark, K.; Swift, R. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161. [Google Scholar] [CrossRef]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of pesticides on soil enzymes: A review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Medronho, B.; Andrade, R.; Vivod, V.; Ostlund, A.; Miguel, M.G.; Lindman, B.; Voncina, B.; Valente, A.J.M. Cyclodextrin-grafted cellulose: Physico-chemical characterization. Carbohydr. Polym. 2013, 93, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Piculell, L.; Lindman, B. Association and segregation in aqueous polymer/polymer, polymer/surfactant, and surfactant/surfactant mixtures: Similarities and differences. Adv. Colloid Interface Sci. 1992, 41, 149–178. [Google Scholar] [CrossRef]
- Painter, P.C.; Veytsman, B.; Kumar, S.; Shenoy, S.; Graf, J.F.; Xu, Y.; Coleman, M.M. Intramolecular Screening Effects in Polymer Mixtures. 1. Hydrogen-Bonded Polymer Blends. Macromolecules 1997, 30, 932–942. [Google Scholar] [CrossRef]
- Sung, S.-S. Dielectric screening effect of electronic polarization and intramolecular hydrogen bonding. Protein Sci. 2017, 26, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.-H.; Cheah, W.-K.; Yeoh, F.-Y. Comparative study on the urea removal by different nanoporous materials. Adsorption 2019, 25, 1169–1175. [Google Scholar] [CrossRef]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J. Chem. Technol. Biotechnol. 2006, 81, 1271–1276. [Google Scholar] [CrossRef]
- Hemine, K.; Skwierawska, A.; Kernstein, A.; Kozłowska-Tylingo, K. Cyclodextrin polymers as efficient adsorbents for removing toxic non-biodegradable pimavanserin from pharmaceutical wastewaters. Chemosphere 2020, 250, 126250. [Google Scholar] [CrossRef] [PubMed]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, F.A.A.; Prins, N. Model Comparisons. In Psychophysics; Kingdom, F.A.A., Prins, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 247–307. ISBN 9780124071568. [Google Scholar]









| Pesticide | AC Source | qm(a) (mg g−1) | RE(b) (%) | Ref. |
|---|---|---|---|---|
| Acetamiprid, Atrazine, Azoxystrobin, Chlorantraniliprole, Difeconazole, Diuron, Imazalil, Pymetrozine, Pyraclostrobin, Thiacloprid, Trifloxyztrobin | Starch | 66.2 (Pyraclostrobin) | >80 | [23] |
| 2,4-dichlorophenoxyacetic acid | Lagenaria vulgaris | 333.3 | 100 | [24] |
| 2,4-dichlorophenoxyacetic acid | Date stone | 238.1 | 91.8 | [25] |
| 4-chloro-2-methylphenoxyacetic acid | Norit 0.8, Aquacarb 207C, Aquacarb 208C, Aquacarb 208EA | 133.6, 117.6, 106.2, 105.3 | n/a | [26] |
| Acetamiprid | Tangerine peels | 35.7 | 92 | [27] |
| Alachlor | Juglans regia | n/a | ≥72 | [28] |
| Ametryn, Aldicarb, Dinoseb, Diuron | Carbon-cloth | 354.6, 421.6, 301.8, 213.1 | n/a | [29] |
| Atrazine | Mangosteen peels | 51 | n/a | [30] |
| Atrazine, deethylatrazine, deisopropylatrazine, simazine | Rayon | 238.1, 303, 119, 370.4 | n/a | [31] |
| Bentazon | CAT, Carbopal | 718 | n/a | [32] |
| Bentazon | Sky fruit husk | 166.7 | ≥70 | [33] |
| Benzimidazole | Arachis hypogaea | n/a | 70 | [34] |
| Carbofuran | Banana stalks | 64 | 97 | [35] |
| Diazinon | NH4Cl-AC | 250 | 97.5 | [36] |
| Endosulfan | Saccharum officinarum | 2.3 | ≥93 | [37] |
| Ethaboxam | Juglans regia | n/a | ≥89 | [38] |
| Furadan, Metribuzin, 2,4-dichlorophenoxyacetic acid | Bamboo | 869, 756.5, 274.7 | 92.3, 93, 96.7 | [39] |
| Imidacloprid | Peach stone | 39.4 | ≥80 | [40] |
| Imidacloprid | Ricinodendron heudelotii | 43.5 | 90 | [41] |
| Hinosan | Rice husk | 81.4 | ≥65 | [42] |
| Methoxychlor, atrazine, methyl parathion | Rubber tire | 112, 104.9, 88.9 | 91, 82, 71 | [43] |
| Adsorbents | SW% | SBET/(m2 g−1) | Dpore(a)/nm |
|---|---|---|---|
| PCD | 55 ± 10 | 11.3 ± 0.2 | 17.7 |
| PCD/AC5% | 50 ± 4 | 36.1 ± 0.7 | 10.8 |
| PCD/AC10% | 33 ± 3 | 33.3 ± 0.9 | 7.91 |
| AC | 1272 ± 38 | 2.05 |
| Ci = 200 ppm | PCD | PCD/AC5% | PCD/AC10% | AC |
| qe,exp (mg g−1) | 10.3 (±0.6) | 14.6 (±0.5) | 24.1 (±0.3) | 37.4 (±0.6) |
| qe (mg g−1) | 10.4 (±0.2) | 15.0 (±0.2) | 24.1 (±0.4) | 37.2 (±0.9) |
| k1 (10−2 min−1) | 75 (±4) | 18.4 (±0.7) | 27.4 (±0.2) | 67 (±5) |
| R2 | 0.9869 | 0.9984 | 0.9953 | 0.9758 |
| AIC | −20.6 | −31.5 | −6.2 | 23.0 |
| qe (mg g−1) | 11.0 (±0.1) | 19.0 (±0.3) | 28.1 (±0.4) | 39.2 (±0.5) |
| k2 (10−2 g mg−1 min−1) | 9.7 (±0.5) | 0.83 (±0.01) | 0.96 (±0.07) | 2.4 (±0.1) |
| R2 | 0.9953 | 0.9983 | 0.9970 | 0.9951 |
| AIC | −33.0 | −28.7 | −9.8 | 2.6 |
| Ci = 500 ppm | PCD | PCD/AC5% | PCD/AC10% | AC |
| qe,exp (mg g−1) | 23 (±3) | 31 (±3) | 32 (±2) | 223.9 (±0.3) |
| qe (mg g−1) | 24.3 (±0.4) | 31.1 (±0.5) | 33.3 (±0.6) | 219 (±2) |
| k1 (10−2 min−1) | 7.1 (±0.7) | 6.5 (±0.5) | 8.4 (±0.8) | 5.7 (±0.2) |
| R2 | 0.9834 | 0.9890 | 0.9821 | 0.9975 |
| AIC | 15.0 | 15.6 | 27.9 | 75.7 |
| qe (mg g−1) | 27.8 (±0.7) | 36.0 (±0.7) | 37.6 (±0.7) | 258(±1) |
| k2 (10−2 g mg−1 min−1) | 0.33 (±0.05) | 0.23 (±0.02) | 0.31 (±0.03) | 0.030 (±0.001) |
| R2 | 0.9844 | 0.9931 | 0.9898 | 0.9996 |
| AIC | 15.9 | 7.6 | 18.2 | 32.21 |
| Ci = 200 ppm | PCD | PCD/AC5% | PCD/AC10% | AC |
| qe,exp (mg g−1) | 10 (±2) | 18.7 (±0.1) | 35.2 (±0.1) | |
| qe (mg g−1) | n/a | 10.2 (±0.3) | 18.8 (±0.4) | 35.6 (±0.2) |
| k1 (10−2 min−1) | n/a | 12 (±1) | 22 (±2) | 21.5 (±0.5) |
| R2 | n/a | 0.9938 | 0.9858 | 0.9990 |
| AIC | n/a | −32.9 | −1.9 | −21.4 |
| qe (mg g−1) | n/a | 14.2 (±0.7) | 22.7 (±0.7) | 43.6 (±0.6) |
| k2 (10−2 g mg−1 min−1) | n/a | 0.6 (±0.1) | 0.9 (±0.1) | 0.44 (±0.02) |
| R2 | n/a | 0.9930 | 0.9883 | 0.9980 |
| AIC | n/a | −29.0 | −3.0 | −9.1 |
| Ci = 500 ppm | PCD | PCD/AC5% | PCD/AC10% | AC |
| qe,exp (mg g−1) | 11 (±4) | 18 (±4) | 27 (±4) | 259 (±1) |
| qe (mg g−1) | 10.4 (±0.8) | 19.2 (±0.8) | 25.4 (±0.7) | 253 (±6) |
| k1 (10−2 min−1) | 5 (±1) | 3.8 (±0.5) | 17 (±3) | 11 (±2) |
| R2 | 0.9171 | 0.9651 | 0.9478 | 0.9608 |
| AIC | 7.6 | 14.8 | 40.1 | 145.5 |
| qe (mg g−1) | 11 (±1) | 23 (±1) | 28.1 (±0.6) | 277 (±1) |
| k2 (10−2 g mg−1 min−1) | 0.7 (±0.3) | 0.18 (±0.04) | 0.65 (±0.09) | 0.081(±0.002) |
| R2 | 0.9384 | 0.9714 | 0.9794 | 0.9992 |
| AIC | 5.8 | 13.0 | 22.6 | 54.0 |
| Model | PCD | PCD/AC5% | PCD/AC10% | AC | |
|---|---|---|---|---|---|
| KF (mg g−1 mg−n Ln) | 0.06 (±0.01) | 0.27 (±0.04) | 1.6 (±0.5) | 21 (±2) | |
| Freundlich | 1/n | 0.93 (±0.03) | 0.77 (±0.03) | 0.48 (±0.05) | 1.7 (±0.3) |
| R2 | 0.9953 | 0.9958 | 0.9651 | 0.9375 | |
| AIC | −18.6 | −8.6 | 13.2 | 26.6 | |
| qm (L mg−1) | 31 (±9) | 48 (±22) | 26 (±2) | 54 (±3) | |
| Sips | KS (10−2 L mg−1) | 0.2 (±0.1) | 0.2 (±0.2) | 2.0 (±0.5) | 97 (±4) |
| 1/n | 1.20 (±0.1) | 1.0 (±0.1) | 1.0 (±0.1) | 5.2 (±0.8) | |
| R2 | 0.9979 | 0.9944 | 0.9945 | 0.9935 | |
| AIC | −27.4 | −5.0 | −0.2 | 13.6 |
| Model | PCD | PCD/AC5% | PCD/AC10% | AC | |
|---|---|---|---|---|---|
| KF (mg g−1 mg−1/n L1/n) | 0.06 (±0.02) | 0.18 (±0.05) | 0.9 (±0.2) | 0.9 (±0.6) | |
| Freundlich | 1/n | 0.75 (±0.07) | 0.70 (±0.05) | 0.51 (±0.05) | 2.4 (±0.4) |
| R2 | 0.9736 | 0.986 | 0.9576 | 0.9265 | |
| AIC | −9.8 | −6.6 | 8.5 | 25.1 | |
| qm (L mg−1) | 9 (±3) | 12 (±3) | 28 (±18) | 57 (±5) | |
| Sips | KS (10−2 L mg−1) | 0.4 (±0.2) | 0.8 (±0.4) | 0.4 (±0.7) | 27 (±1) |
| 1/n | 1.3 (±0.3) | 1.3 (±0.4) | 0.7 (±0.3) | 8 (±1) | |
| R2 | 0.9871 | 0.9804 | 0.9527 | 0.9865 | |
| AIC | −14.6 | −3.4 | 13.0 | 17.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utzeri, G.; Verissimo, L.; Murtinho, D.; Pais, A.A.C.C.; Perrin, F.X.; Ziarelli, F.; Iordache, T.-V.; Sarbu, A.; Valente, A.J.M. Poly(β-cyclodextrin)-Activated Carbon Gel Composites for Removal of Pesticides from Water. Molecules 2021, 26, 1426. https://doi.org/10.3390/molecules26051426
Utzeri G, Verissimo L, Murtinho D, Pais AACC, Perrin FX, Ziarelli F, Iordache T-V, Sarbu A, Valente AJM. Poly(β-cyclodextrin)-Activated Carbon Gel Composites for Removal of Pesticides from Water. Molecules. 2021; 26(5):1426. https://doi.org/10.3390/molecules26051426
Chicago/Turabian StyleUtzeri, Gianluca, Luis Verissimo, Dina Murtinho, Alberto A. C. C. Pais, F. Xavier Perrin, Fabio Ziarelli, Tanta-Verona Iordache, Andrei Sarbu, and Artur J. M. Valente. 2021. "Poly(β-cyclodextrin)-Activated Carbon Gel Composites for Removal of Pesticides from Water" Molecules 26, no. 5: 1426. https://doi.org/10.3390/molecules26051426
APA StyleUtzeri, G., Verissimo, L., Murtinho, D., Pais, A. A. C. C., Perrin, F. X., Ziarelli, F., Iordache, T. -V., Sarbu, A., & Valente, A. J. M. (2021). Poly(β-cyclodextrin)-Activated Carbon Gel Composites for Removal of Pesticides from Water. Molecules, 26(5), 1426. https://doi.org/10.3390/molecules26051426