Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis
Abstract
:1. Introduction
2. Results
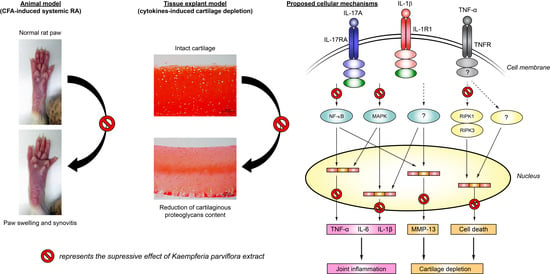
2.1. Attenuating Effect of KP Extract on Rat Arthritis Induced by CFA
2.2. Chondroprotecting Effects of KP Extract on Cartilage Explant Degradation
2.3. Suppressing Effect of KP Extract and Its Major Components on Arthritis-Related Gene Expressions and Signaling Pathways in SW1353 Cells
2.4. Protecting Effects of KP Extract against Cell Death Pathways
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Plant Extraction and Preparation
4.3. Complete Freund’s Adjuvant-Induced Rat Model
4.4. Assessments of Arthritis Severity Scores
4.5. Histopathological Examination
4.6. Porcine Cartilage Explant Model
4.7. Assessments of Cartilage Degradation
4.8. Cell Culture Model
4.9. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Western Blot Analysis
4.11. Flow Cytometric Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Woetzel, D.; Huber, R.; Kupfer, P.; Pohlers, D.; Pfaff, M.; Driesch, D.; Häupl, T.; Koczan, D.; Stiehl, P.; Guthke, R.; et al. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res. Ther. 2014, 16, R84. [Google Scholar] [CrossRef] [Green Version]
- Laev, S.S.; Salakhutdinov, N.F. Anti-arthritic agents: Progress and potential. Bioorganic Med. Chem. 2015, 23, 3059–3080. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alunno, A.; Carubbi, F.; Giacomelli, R.; Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: New players and therapeutic targets. BMC Rheumatol. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jeon, J.; Shin, M.; Won, Y.; Lee, M.; Kwak, J.-S.; Lee, G.; Rhee, J.; Ryu, J.-H.; Chun, C.-H.; et al. Regulation of the Catabolic Cascade in Osteoarthritis by the Zinc-ZIP8-MTF1 Axis. Cell 2014, 156, 730–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, G.; Bonaventura, P.; Lavocat, F.; Miossec, P. IL-17A and TNF-α Increase the Expression of the Antiapoptotic Adhesion Molecule Amigo-2 in Arthritis Synoviocytes. Front. Immunol. 2016, 7, 254. [Google Scholar] [CrossRef] [Green Version]
- Del Grossi Moura, M.; Cruz Lopes, L.; Silva, M.T.; Barberato-Filho, S.; Motta, R.H.L.; Bergamaschi, C.C. Use of steroid and nonsteroidal anti-inflammatories in the treatment of rheumatoid arthritis: Systematic review protocol. Medicine 2018, 97, e12658. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Sato, K.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ichinose, K.; Aburada, M.; Ochiai, W.; Sugiyama, K.; et al. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2017, 72, 136–144. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Liu, H.; Yang, K.; Liu, Z.; Huang, H. 5,7,3′,4′-Tetramethoxyflavone exhibits chondroprotective activity by targeting beta-catenin signaling in vivo and in vitro. Biochem. Biophys. Res. Commun. 2014, 452, 682–688. [Google Scholar] [CrossRef]
- Yuan, X.; Li, L.; Shi, W.; Liu, H.; Huang, X.; Liu, Z.; Wu, L. TMF protects chondrocytes from ER stress-induced apoptosis by down-regulating GSK-3beta. Biomed. Pharmacother. 2017, 89, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Kongdang, P.; Jaitham, R.; Thonghoi, S.; Kuensaen, C.; Pradit, W.; Ongchai, S. Ethanolic extract of Kaempferia parviflora inter-rupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine 2019, 59, 152755. [Google Scholar] [CrossRef]
- Gynther, G.W.; Dijkgraaf, L.C.; Reinholt, F.P.; Holmlund, A.B.; Liem, R.S.; de Bont, L.G. Synovial inflammation in ar-throscopically obtained biopsy specimens from the temporomandibular joint: A review of the literature and a proposed histologic grading system. J. Oral Maxillofac. Surg. 1998, 56, 1281–1286; discussion 1287. [Google Scholar] [CrossRef]
- Saokaew, S.; Wilairat, P.; Raktanyakan, P.; Dilokthornsakul, P.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Clinical Effects of Krachaidum (Kaempferia parviflora): A Systematic Review. J. Evid. Based Integr. Med. 2016, 22, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid. Based Complement. Altern. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef] [Green Version]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, Bioavailability, Tissue Distribution, Excretion, and Metabolite Identification of Methoxyflavones in Kaempferia parviflora Extract in Rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef] [Green Version]
- Chivapat, S.; Chavalittumrong, P.; Attawish, A.; Rungsipipat, A. Chronic toxicity study of Kaempferia parviflora Wall ex. extract. Thai. J. Vet. Med. 2010, 40, 377–383. [Google Scholar]
- Mossiat, C.; Laroche, D.; Prati, C.; Pozzo, T.; Demougeot, C.; Marie, C. Association between arthritis score at the onset of the disease and long-term locomotor outcome in adjuvant-induced arthritis in rats. Arthritis Res. Ther. 2015, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Houshmand, G.; Mansouri, M.T.; Naghizadeh, B.; Hemmati, A.A.; Hashemitabar, M. Potentiation of indomethacin-induced anti-inflammatory response by pioglitazone in carrageenan-induced acute inflammation in rats: Role of PPARgamma re-ceptors. Int. Immunopharmacol. 2016, 38, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.J.; Khan NA, K.; Asmawi MZ, B.; Mahmud, R.; Vikneswaran, A.; Murugaiyah, L. In vivo anti-arthritic and anti-nociceptive effects of ethanol extract of Moringa oleifera leaves on complete Freund’s adjuvant (CFA)-induced arthritis in rats. Integr Med. Res. 2018, 7, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hanprasertpong, N.; Teekachunhatean, S.; Chaiwongsa, R.; Ongchai, S.; Kunanusorn, P.; Sangdee, C.; Panthong, A.; Bunteang, S.; Nathasaen, N.; Reutrakul, V. Analgesic, anti-inflammatory, and chondroprotective activities of Cryptolepis buchanani ex-tract: In vitro and in vivo studies. Biomed. Res. Int. 2014, 978582. [Google Scholar] [CrossRef] [Green Version]
- Chabaud, M.; Lubberts, E.; Joosten, L.; Berg, W.V.D.; Miossec, P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001, 3, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wu, L.; Li, L.; Chen, S. Monotropein exerts protective effects against IL-1beta-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int. Immunopharmacol. 2014, 23, 575–580. [Google Scholar] [CrossRef]
- Gebauer, M.; Saas, J.; Sohler, F.; Haag, J.; Soder, S.; Pieper, M.; Bartnik, E.; Beninga, J.; Zimmer, R.; Aigner, T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthr. Cartil. 2005, 13, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Lee, E.J.; Park, W.D.; Kim, J.B.; Kim, E.O.; Choi, S.W. Anti-inflammatory and anti-osteoarthritis effects of fermented Achyranthes japonica Nakai. J. Ethnopharmacol. 2012, 142, 634–641. [Google Scholar] [CrossRef]
- Park, J.U.; Kim, S.J.; Na, C.S.; Choi, C.H.; Seo, C.S.; Son, J.K.; Kang, B.Y.; Kim, Y.R. Chondroprotective and anti-inflammatory effects of ChondroT, a new complex herbal medication. BMC Complement. Altern Med. 2016, 16, 213. [Google Scholar] [CrossRef] [Green Version]
- Ruangsuriya, J.; Budprom, P.; Viriyakhasem, N.; Kongdang, P.; Chokchaitaweesuk, C.; Sirikaew, N.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. Suppression of Cartilage Degradation by Zingerone Involving the p38 and JNK MAPK Signaling Pathway. Planta Med. 2016, 83, 268–276. [Google Scholar] [CrossRef]
- Yazici, Y.; Curtis, J.R.; Ince, A.; Baraf, H.; Malamet, R.L.; Teng, L.L.; Kavanaugh, A. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: The ROSE study. Ann. Rheum. Dis. 2011, 71, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.; Knauper, V.; Atkinson, S.; Butler, G.; English, W.; Hutton, M.; Stracke, J.; Clark, I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002, 4, S39–S49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchetverikov, I.; Lohmander, L.S.; Verzijl, N.; Huizinga, T.W.J.; Tekoppele, J.M.; Hanemaaijer, R.; DeGroot, J. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann. Rheum. Dis. 2005, 64, 694–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, K. Osteoarthritis: Zinc linked with osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. 2017, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Kim, D.; Lee, C.H.; Lee, M.S.; Chun, C.-H.; Jin, E.-J. MicroRNA-488 regulates zinc transporter SLC39A8/ZIP8 during pathogenesis of osteoarthritis. J. Biomed. Sci. 2013, 20, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malemud, C.J. Intracellular Signaling Pathways in Rheumatoid Arthritis. J. Clin. Cell. Immunol. 2013, 4, 160. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.M.; Zhao, X.; Yang, T.; Duan, Y.H.; Sun, Y.B.; Zhao, W.J.; Tian, M.Q. Exploring the key genes and pathways of osteoarthritis in knee cartilage in a rat model using gene expression profiling. Yonsei Med. J. 2018, 59, 760–768. [Google Scholar] [CrossRef]
- Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets 2007, 8, 305–313. [Google Scholar] [CrossRef]
- Li, H.; Wan, A. Apoptosis of rheumatoid arthritis fibroblast-like synoviocytes: Possible roles of nitric oxide and the thioredoxin 1. Mediat. Inflamm. 2013, 953462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Koh, J.H.; Leng, L.; Kim, W.-U.; Bucala, R. Review: The Tumor-Like Phenotype of Rheumatoid Synovium: Molecular Profiling and Prospects for Precision Medicine. Arthritis Rheumatol. 2018, 70, 637–652. [Google Scholar] [CrossRef]
- Malemud, C.J. Pharmacologic Interventions for Preventing Chondrocyte Apoptosis in Rheumatoid Arthritis and Osteoarthritis. Drug Discov. Concepts Market 2018, 45, 77. [Google Scholar]
- Liu, Y.L.; Lin, H.M.; Zou, R.; Wu, J.C.; Han, R.; Raymond, L.N.; Reid, P.F.; Qin, Z.H. Suppression of complete Freund’s adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharm. Sin. 2009, 30, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Megraw, R.E. Reaction of reduced nicotinamide adenine dinucleotide with 2,4-dinitrophenylhydrazine in serum lactate dehydrogenase assays. Am. J. Clin. Pathol. 1971, 56, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Et Biophys. Acta (Bba) Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]






| Group | Synovial Lining Layers (Point) | Vascularity (Point) | Inflammatory Cells (Point) |
|---|---|---|---|
| Normal | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.7 ± 0.7 |
| Arthritis | 1.5 ± 0.3 * | 1.8 ± 0.5 * | 6.8 ± 2.0 * |
| Indo | 1.3 ± 0.3 * | 0.6 ± 0.2 # | 2.6 ± 0.7 # |
| KP150 | 0.4 ± 0.2 | 0.6 ± 0.2 # | 1.6 ± 0.7 # |
| KP300 | 0.4 ± 0.2 | 0.8 ± 0.2 | 1.6 ± 0.4 # |
| Normal | Arthritis | Indo | KP150 | KP300 | |
|---|---|---|---|---|---|
| Organ Weights | |||||
| Liver (g) | 5.20 ± 0.19 | 4.94 ± 0.41 | 5.18 ± 0.28 | 4.38 ± 0.10 | 5.11 ± 0.17 |
| Spleen (g) | 0.42 ± 0.01 | 0.41 ± 0.02 | 0.60 ± 0.07 * | 0.37 ± 0.01 | 0.42 ± 0.01 |
| Kidney (g) | 1.37 ± 0.00 | 1.33 ± 0.04 | 1.24 ± 0.03 | 1.17 ± 0.02 * | 1.35 ± 0.06 |
| Thymus (g) | 0.22 ± 0.03 | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.23 ± 0.02 |
| Hematological Parameters | |||||
| White blood cell (103/μL) | 2.49 ± 0.42 | 2.46 ± 0.30 | 4.09 ± 0.49 | 2.55 ± 0.42 | 3.00 ± 0.39 |
| Red blood cell (106/μL) | 7.03 ± 0.12 | 7.09 ± 0.14 | 5.29 ± 0.34 * | 7.40 ± 0.14 | 7.32 ± 0.12 |
| Hemoglobin (g/dL) | 13.2 ± 0.2 | 13.5 ± 0.2 | 10.3 ± 0.6 * | 13.9 ± 0.2 | 13.7 ± 0.2 |
| Hematocrit (%) | 40.5 ± 0.7 | 40.9 ± 0.6 | 33.1 ± 1.7 * | 41.8 ± 0.5 | 41.6 ± 0.7 |
| Platelet (103/μL) | 679 ± 61 | 695 ± 18 | 1044 ± 147 | 677 ± 20 | 571 ± 38 |
| Blood Chemistry Tests | |||||
| Glucose (mg/dL) | 130 ± 8 | 110 ± 9 | 84 ± 11 | 116 ± 7 | 122 ± 12 |
| Aspartate aminotransferase (U/L) | 110 ± 2 | 122 ± 15 | 122 ± 12 | 120 ± 11 | 106 ± 22 |
| Alanine aminotransferase (U/L) | 44 ± 5 | 48 ± 2 | 33 ± 3 | 37 ± 1 | 46 ± 3 |
| Alkaline phosphatase (U/L) | 106 ± 5 | 113 ± 5 | 139 ± 34 | 114 ± 5 | 131 ± 10 |
| Blood urea nitrogen (mg/dL) | 16.1 ± 1.2 | 19.6 ± 2.0 | 18.9 ± 1.0 | 16.3 ± 0.8 | 17.8 ± 1.0 |
| Creatinine (mg/dL) | 0.56 ± 0.03 | 0.58 ± 0.02 | 0.54 ± 0.01 | 0.54 ± 0.01 | 0.54 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ongchai, S.; Chiranthanut, N.; Tangyuenyong, S.; Viriyakhasem, N.; Kongdang, P. Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis. Molecules 2021, 26, 1527. https://doi.org/10.3390/molecules26061527
Ongchai S, Chiranthanut N, Tangyuenyong S, Viriyakhasem N, Kongdang P. Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis. Molecules. 2021; 26(6):1527. https://doi.org/10.3390/molecules26061527
Chicago/Turabian StyleOngchai, Siriwan, Natthakarn Chiranthanut, Siriwan Tangyuenyong, Nawarat Viriyakhasem, and Patiwat Kongdang. 2021. "Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis" Molecules 26, no. 6: 1527. https://doi.org/10.3390/molecules26061527
APA StyleOngchai, S., Chiranthanut, N., Tangyuenyong, S., Viriyakhasem, N., & Kongdang, P. (2021). Kaempferia parviflora Extract Alleviated Rat Arthritis, Exerted Chondroprotective Properties In Vitro, and Reduced Expression of Genes Associated with Inflammatory Arthritis. Molecules, 26(6), 1527. https://doi.org/10.3390/molecules26061527