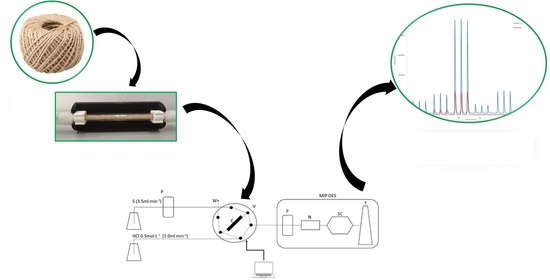
On-Line Preconcentration and Simultaneous Determination of Cu and Mn in Water Samples Using a Minicolumn Packed with Sisal Fiber by MIP OES
Abstract
:1. Introduction
2. Results and Discussion
2.1. Flow Injection System Coupled to MIP OES
2.2. Optimization
2.3. Validation
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Preconcentration Minicolum
3.3. Samples
3.4. Optimization and Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fernández, S.; Villanueva, U.; de Diego, A.; Arana, G.; Madariaga, J.M. Monitoring trace elements (Al, As, Cr, Cu, Fe, Mn, Ni and Zn) in deep and surface waters of the estuary of the Nerbioi-Ibaizabal River (Bay of Biscay, Basque Country). J. Mar. Syst. 2008, 72, 332–341. [Google Scholar] [CrossRef]
- Tzempelikou, E.; Zeri, C.; Iliakis, S.; Paraskevopoulou, V. Cd, Co, Cu, Ni, Pb, Zn in coastal and transitional waters of Greece and assessment of background concentrations: Results from 6 years implementation of the Water Framework Directive. Sci. Total Environ. 2021, 774, 145177. [Google Scholar] [CrossRef]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Vázquez-Mejía, G.; Solache-Ríos, M.; Fonseca-Montes de Oca, G.; Trujillo-Flores, E. The corrosive nature of manganese in drinking wáter. Sci. Total Environ. 2013, 447C, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tian, Y.; Guo, Y.; Shan, J.; Liu, R. Manganese release from corrosion products of cast iron pipes in drinking water distribution systems: Effect of water temperature, pH, alkalinity, SO42− concentration and disinfectants. Chemosphere 2021, 262, 127904. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Secondary Drinking Water Regulations Guidance for Nuisance Chemicals EPA-816F10-079. Available online: http://www.epa.gov/safewater (accessed on 1 March 2021).
- Thomas, R. Practical Guide to ICP-MS: A Tutorial for Beginners, (Practical Spectroscopy), 3rd ed.; Edición Kindle; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Williams, C.B.; Amais, R.S.; Fontoura, B.M.; Jones, B.T.; Nóbrega, J.A.; Donati, G.L. Recent developments in microwave-induced plasma optical emission spectrometry and applications of a commercial Hammer-cavity instrument. Trends Anal. Chem. 2019, 116, 151–157. [Google Scholar] [CrossRef]
- Fang, Z. Flow Injection Separation and Preconcentration; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Liang, H.D.; Han, D.M. Multi-Walled Carbon Nanotubes as Sorbent for Flow Injection On-Line Microcolumn Preconcentration Coupled with Flame Atomic Absorption Spectrometry for Determination of Cadmium and Copper. Anal. Lett. 2006, 39, 2285–2295. [Google Scholar] [CrossRef]
- Pistón, M.; Silva, J.; Pérez-Zambra, R.; Knochen, M. Determination of total selenium by multicommutated-flow hydride generation atomic absorption spectrometry. Application to cow’s milk and infant formulae. Anal. Methods 2009, 1, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Pistón, M.; Silva, J.; Pérez-Zambra, R.; Dol, I.; Knochen, M. Automated method for determination of total arsenic and selenium in natural and drinking water by HG-AAS. Environ. Geochem. Health 2012, 34, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Pistón, M.; Knochen, M. Determination of total selenium in infant formulas. Comparison of the performance of FIA and MCFA flow systems. Int. J. Anal. Chem. 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.G.; Lemos, V.A.; Melo Coelho, L.; Rocha, F.R.P. Applications of biosorbents in atomic spectrometry. Appl. Spectrosc. Rev. 2016, 51, 36–72. [Google Scholar] [CrossRef]
- Shih, T.T.; Chen, J.Y.; Luo, Y.T.; Lin, C.H.; Liu, Y.H.; Su, Y.A.; Chao, P.C.; Sun, Y.C. Development of a titanium dioxide-assisted preconcentration/on-site vapor-generation chip hyphenated with inductively coupled plasma-mass spectrometry for online determination of mercuric ions in urine samples. Anal. Chim. Acta 2019, 1063, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hajeeth, T.; Vijayalakshmi, K.; Gomathi, T.; Sudha, P.N. Removal of Cu(II) and Ni(II) using cellulose and extracted from sisal fiber cellulose-g-acrylic acid copolymer. Int. J. Biol. Macromol. 2013, 62, 59–65. [Google Scholar] [CrossRef] [PubMed]
- de Souza Días, F.; Santana Bonsucesso, J.; Suzart Alves, L.; Correia da Silva Filho, D.; Spínola Costa, A.C.; Lopes dos Santos, W.N. Development and optimization of analytical method for the determination of cadmium from mineral water samples by off-line solid phase extraction system using sisal fiber loaded TAR by FAAS. Microchem. J. 2013, 106, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Giri Dev, V.R.; Venugopal, J.R.; Kumar, T.S.; Miranda, L.R.; Ramakrishna, S. Agave sisalana, a biosorbent for the adsorption of Reactive Red 120 from aqueous solution. J. Text. Inst. 2010, 101, 414–422. [Google Scholar] [CrossRef]
- de Souza Días, F.; Bonsucesso, J.S.; Oliveira, L.C.; dos Santos, W.N.L. Preconcentration and determination using of copper in tobacco leaves simples by minicolumn of sisal fiber (Agave sisalana) loaded with Alizarin fluorine blue by FAAS. Talanta 2012, 89, 276–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjos, S.L.; Almeida, J.S.; Teixeira, L.S.G.; da Silva, A.C.M.; Santos, A.P.; Queiroz, A.F.S.; Ferreira, S.L.C.; Mattedi, S. Determination of Cu, Ni, Mn and Zn in diesel oil samples using energy dispersive X-ray fluorescence spectrometry after solid phase extraction using sisal fiber. Talanta 2021, 225, 121910. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.C.G.; De Souza, J.V.T.M.; Tarley, C.R.T.; Caetano, J.; Cardoso Dragunski, D. Copper Ions Adsorption from Aqueous Medium Using the Biosorbent Sugarcane Bagasse In Natura and Chemically Modified. Water Air Soil Pollut. 2011, 216, 351–359. [Google Scholar] [CrossRef]
- Saramasan, C.; Drăghici, S.; Daina, L. Identification, Communication and management of risks relating to drinking water pollution in Bihor County. Environ. Res. Eng. Manag. 2008, 7, 769–777. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Torino, Italy, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- Machado, I.; Mondutey, S.; Patorino, N.; Arce, V.; Pistón, M. A green analytical method for the determination of Cu, Fe, Mn, and Zn in wheat flour using total reflection X-ray fluorescence. J. Anal. At. Spectrosc. 2018, 33, 1264–1268. [Google Scholar] [CrossRef]
- Welcome to Phyton. Available online: www.phyton.org (accessed on 1 March 2021).
- Massart, M.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier Science: Amsterdam, The Netherlands, 1997. [Google Scholar]




| Selected by the Commercial Software 1 | Selected for This Method | |||
|---|---|---|---|---|
| Cu | Mn | Cu | Mn | |
| Viewing position | 10 | −10 | 10 | −10 |
| Nebulizer flow (L min−1) | 0.65 | 0.95 | 0.9 | 0.9 |
| Exp# | Preconcentration Time (s) | pH | (HCl) (mol L−1) | Cu (Peak Height 1) | Mn (Peak Height 1) |
|---|---|---|---|---|---|
| 1 | 30 | 4 | 0.2 | 674 | 127 |
| 2 | 30 | 4 | 0.5 | 589 | 114 |
| 3 | 30 | 6 | 0.2 | 401 | 99 |
| 4 | 30 | 6 | 0.5 | 363 | 93 |
| 5 | 60 | 5.5 | 0.3 | 1289 | 226 |
| 6 | 90 | 4 | 0.2 | 1872 | 277 |
| 7 | 90 | 4 | 0.5 | 1798 | 258 |
| 8 | 90 | 6 | 0.2 | 1079 | 223 |
| 9 | 90 | 6 | 0.5 | 1049 | 198 |
| 10 | 90 | 5.5 | 0.3 | 1854 | 298 |
| 11 | 90 | 5.5 | 0.5 | 1925 | 321 |
| Cu | Mn | |
|---|---|---|
| Linear range (µg L−1) | 12–500 | 30–500 |
| LOD (µg L−1) | 3.7 | 9.0 |
| LOQ (µg L−1) | 12 | 30 |
| Trueness (%R) * | 96.5 | 98.0 |
| Precision (%RSD, n = 6) ** | 4.3 | 2.6 |
| Precision (%RSD, n = 6) *** | 9 | 10 |
| Developed Method (µg L−1) | ETAAS (µg L−1) | t Value | ||||
|---|---|---|---|---|---|---|
| Sample | Cu | Mn | Cu | Mn | Cu | Mn |
| Well water 1 | 93 ± 9 | ND | 82 ± 1 | <LOQ | 2.44 | -- |
| Well water 2 | 79 ± 3 | ND | 81 ± 1 | <LOQ | 1.50 | -- |
| Well water 3 | 108 ± 4 | 175 ± 9 | 114 ± 2 | 161 ± 1 | −1.07 | 2.2 |
| Well water 4 | 124 ± 11 | ND | 113 ± 1 | <LOQ | 2.03 | -- |
| Well water 5 | 61 ± 5 | ND | 55 ± 2 | <LOQ | 1.14 | -- |
| Well water 6 | 35.4 ± 2.4 | 57.5 ± 1.3 | 39.5 ± 5.0 | 56.9 ± 1.0 | −0.44 | 0.37 |
| Tap water | 106 ± 1 | ND | 105 ± 1 | <LOQ | 0.68 | -- |
| Rainwater | ND | ND | <LOQ | <LOQ | -- | -- |
| Stage | Temperature (°C) | Hold Time (s) | Ramp Rate (°C s−1) | Internal Ar Flow (mL min−1) |
|---|---|---|---|---|
| Drying | 110 | 1 | 30 | 250 |
| Drying | 130 | 15 | 20 | 250 |
| Pyrolysis | 1100(Cu)/1050(Mn) | 10 | 20 | 250 |
| Atomization | 2300(Cu)/2100(Mn) | 0 | 5 | 0 |
| Cleaning | 2600 | 1 | 5 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Pistón, M. On-Line Preconcentration and Simultaneous Determination of Cu and Mn in Water Samples Using a Minicolumn Packed with Sisal Fiber by MIP OES. Molecules 2021, 26, 1662. https://doi.org/10.3390/molecules26061662
Silva J, Pistón M. On-Line Preconcentration and Simultaneous Determination of Cu and Mn in Water Samples Using a Minicolumn Packed with Sisal Fiber by MIP OES. Molecules. 2021; 26(6):1662. https://doi.org/10.3390/molecules26061662
Chicago/Turabian StyleSilva, Javier, and Mariela Pistón. 2021. "On-Line Preconcentration and Simultaneous Determination of Cu and Mn in Water Samples Using a Minicolumn Packed with Sisal Fiber by MIP OES" Molecules 26, no. 6: 1662. https://doi.org/10.3390/molecules26061662
APA StyleSilva, J., & Pistón, M. (2021). On-Line Preconcentration and Simultaneous Determination of Cu and Mn in Water Samples Using a Minicolumn Packed with Sisal Fiber by MIP OES. Molecules, 26(6), 1662. https://doi.org/10.3390/molecules26061662