Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
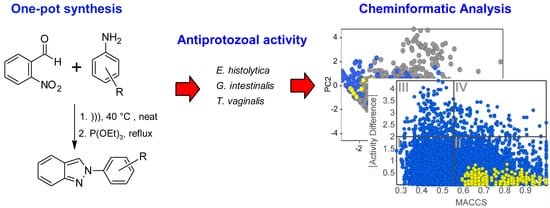
2.1. Synthesis of 2-Phenyl-2H-Indazole Derivatives
2.2. Antiprotozoal Activity
2.3. Cheminformatic Analysis
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Chemistry
3.2.1. General Procedure for the One-Pot Synthesis of 2-Phenyl-2H-Indazole Derivatives 1–6, 9–13, 16, 17, 19 and 20
3.2.2. General Procedure for O-Demethylation
3.2.3. General Procedure for the Hydrolysis of Ester Derivatives
3.2.4. Synthesis of Compound 18 and 22
3.3. Antiprotozoal Activity Assays
3.4. Cheminformatic Studies
3.4.1. Molecular Databases
3.4.2. Property Space
3.4.3. Structure-Activity Similarity (SAS) Maps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scott, L.J. Niraparib: First global approval. Drugs 2017, 77, 1029–1034. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First global approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Hugo, C.; Alejandra, G.; Mercedes, G.; Vicente, J.A.; Carmen, O.d.O. Pharmacological properties of indazole derivatives: Recent developments. Mini. Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole derivatives and their therapeutic applications: A patent review (2013-2017). Expert. Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Liang, C.-G.; Zhang, W.-H. Recent advances in indazole-containing derivatives: Synthesis and biological perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef] [Green Version]
- López-Vallejo, F.; Castillo, R.; Yépez-Mulia, L.; Medina-Franco, J.L. Benzotriazoles and indazoles are scaffolds with biological activity against Entamoeba histolytica. J. Biomol. Screen. 2011, 16, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Villanueva, J.; Yépez-Mulia, L.; González-Sánchez, I.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Sainz-Espuñes, T.D.R.; Cerbón, M.A.; Rodríguez-Villar, K.; Rodríguez-Vicente, A.K.; Cortés-Gines, M.; et al. synthesis and biological evaluation of 2H-indazole derivatives: Towards antimicrobial and anti-inflammatory dual agents. Molecules 2017, 22, 1864. [Google Scholar] [CrossRef] [Green Version]
- Marrero-Ponce, Y.; Meneses-Marcel, A.; Castillo-Garit, J.A.; Machado-Tugores, Y.; Escario, J.A.; Barrio, A.G.; Pereira, D.M.; Nogal-Ruiz, J.J.; Arán, V.J.; Martínez-Fernández, A.R.; et al. Predicting antitrichomonal activity: A computational screening using atom-based bilinear indices and experimental proofs. Bioorg. Med. Chem. 2006, 14, 6502–6524. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in clinical manifestation, pathogenesis, and vaccine development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef] [Green Version]
- Cacciò, S.; Sprong, H. Epidemiology of giardiasis in humans. In Giardia a Model Organism, 1st ed.; Lujan, H.D., Svärd, S., Eds.; Springer: Vienna, Austria, 2011; Volume 1, pp. 17–28. [Google Scholar]
- Hodges, K.; Gill, R. Infectious diarrhea: Cellular and molecular mechanisms. Gut. Microb. 2010, 1, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Bala, V.; Chhonker, Y.S. Recent developments in anti-Trichomonas research: An update review. Eur. J. Med. Chem. 2018, 143, 232–243. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548P–562P. [Google Scholar] [CrossRef]
- Schwebke, J.R. Update of trichomoniasis. Sex. Transm. Infect. 2002, 78, 378–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upcroft, P.; Upcroft, J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upcroft, J.A.; Upcroft, P. Drug susceptibility testing of anaerobic protozoa. Antimicrob. Agents Chemother. 2001, 45, 1810–1814. [Google Scholar] [CrossRef] [Green Version]
- García, C.G.; Marchat, L.A.; López-Cánovas, L.; Ishiwara, D.G.P.; Rodríguez, M.A.; Orozco, E. Drug resistance mechanisms in Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, and opportunistic anaerobic protozoa. In Antimicrobial Drug Resistance: Mechanisms of Drug Resistance, 2nd ed.; Mayers, D.L., Sobel, J.D., Ouellette, M., Kaye, K.S., Marchaim, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, pp. 613–628. [Google Scholar]
- Leitsch, D. Drug resistance in the microaerophilic parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, D.E. Solvent-free sonochemistry: Sonochemical organic synthesis in the absence of a liquid medium. Beilstein J. Org. Chem. 2017, 13, 1850–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadogan, J.; Mackie, R. 2-Phenylindazole. Org. Synth. 1968, 48, 113. [Google Scholar]
- Schaeffer, L. The role of functional groups in drug–receptor interactions. In The Practice of Medicinal Chemistry, 4th ed.; Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: San Diego, CA, USA, 2008; Volume 1, pp. 359–378. [Google Scholar]
- Navarrete-Vazquez, G.; Rojano-Vilchis, M.D.; Yepez-Mulia, L.; Melendez, V.; Gerena, L.; Hernandez-Campos, A.; Castillo, R.; Hernandez-Luis, F. Synthesis and antiprotozoal activity of some 2-(trifluoromethyl)-1H-benzimidazole bioisosteres. Eur. J. Med. Chem. 2006, 41, 135–141. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Yepez, L.; Hernandez-Campos, A.; Tapia, A.; Hernandez-Luis, F.; Cedillo, R.; Gonzalez, J.; Martinez-Fernandez, A.; Martinez-Grueiro, M.; Castillo, R. Synthesis and antiparasitic activity of albendazole and mebendazole analogues. Bioorg. Med. Chem. 2003, 11, 4615–4622. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Yepez-Mulia, L.; Cedillo-Rivera, R.; Tapia, A.; Vilpo, L.; Vilpo, J.; Kazimierczuk, Z. Synthesis, antiprotozoal and anticancer activity of substituted 2-trifluoromethyl- and 2-pentafluoroethylbenzimidazoles. Eur. J. Med. Chem. 2002, 37, 973–978. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Yepez-Mulia, L.; Tapia, A.; Cedillo-Rivera, R.; Laudy, A.E.; Starosciak, B.J.; Kazimierczuk, Z. Synthesis, and antiprotozoal and antibacterial activities of S-substituted 4,6-dibromo- and 4,6-dichloro-2-mercaptobenzimidazoles. Eur. J. Pharm. Sci. 2004, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Padilla, D.; Rodriguez-Morales, S.; Hernandez-Campos, A.; Hernandez-Luis, F.; Yepez-Mulia, L.; Tapia-Contreras, A.; Castillo, R. Synthesis and antiprotozoal activity of novel 1-methylbenzimidazole derivatives. Bioorg. Med. Chem. 2009, 17, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Vazquez, G.; Cedillo, R.; Hernandez-Campos, A.; Yepez, L.; Hernandez-Luis, F.; Valdez, J.; Morales, R.; Cortes, R.; Hernandez, M.; Castillo, R. Synthesis and antiparasitic activity of 2-(trifluoromethyl)-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 187–190. [Google Scholar] [CrossRef]
- Hernandez-Luis, F.; Hernandez-Campos, A.; Castillo, R.; Navarrete-Vazquez, G.; Soria-Arteche, O.; Hernandez-Hernandez, M.; Yepez-Mulia, L. Synthesis and biological activity of 2-(trifluoromethyl)-1H-benzimidazole derivatives against some protozoa and Trichinella Spiralis. Eur. J. Med. Chem. 2010, 45, 3135–3141. [Google Scholar] [CrossRef]
- Valdez, J.; Cedillo, R.; Hernandez-Campos, A.; Yepez, L.; Hernandez-Luis, F.; Navarrete-Vazquez, G.; Tapia, A.; Cortes, R.; Hernandez, M.; Castillo, R. Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 2221–2224. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Medina-Franco, J.L.; Caulfield, T.R.; Hernández-Campos, A.; Hernández-Luis, F.; Yépez-Mulia, L.; Castillo, R. Comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) of some benzimidazole derivatives with trichomonicidal activity. Eur. J. Med. Chem. 2011, 46, 3499–3508. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 4221–4224. [Google Scholar] [CrossRef]
- Soria-Arteche, O.; Hernández-Campos, A.; Yépez-Mulia, L.; Trejo-Soto, P.J.; Hernández-Luis, F.; Gres-Molina, J.; Maldonado, L.A.; Castillo, R. Synthesis and antiprotozoal activity of nitazoxanide–N-methylbenzimidazole hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 6838–6841. [Google Scholar] [CrossRef]
- Lopez-Vallejo, F.; Medina-Franco, J.L.; Hernandez-Campos, A.; Rodriguez-Morales, S.; Yepez, L.; Cedillo, R.; Castillo, R. Molecular modeling of some 1H-benzimidazole derivatives with biological activity against Entamoeba Histolytica: A comparative molecular field analysis study. Bioorg. Med. Chem. 2007, 15, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Covarrubias, C.; Vilchis-Reyes, M.A.; Yépez-Mulia, L.; Sánchez-Díaz, R.; Navarrete-Vázquez, G.; Hernández-Campos, A.; Castillo, R.; Hernández-Luis, F. Exploring the interplay of physicochemical properties, membrane permeability and giardicidal activity of some benzimidazole derivatives. Eur. J. Med. Chem. 2012, 52, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Flores-Carrillo, P.; Velázquez-López, J.M.; Aguayo-Ortiz, R.; Hernández-Campos, A.; Trejo-Soto, P.J.; Yépez-Mulia, L.; Castillo, R. Synthesis, antiprotozoal activity, and chemoinformatic analysis of 2-(methylthio)-1H-benzimidazole-5-carboxamide derivatives: Identification of new selective giardicidal and trichomonicidal compounds. Eur. J. Med. Chem. 2017, 137, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2011, 40, D1100–D1107. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Villanueva, J.; Santos, R.; Hernández-Campos, A.; Giulianotti, M.A.; Castillo, R.; Medina-Franco, J.L. Towards a systematic characterization of the antiprotozoal activity landscape of benzimidazole derivatives. Bioorg. Med. Chem. 2010, 18, 7380–7391. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Santos, R.; Hernández-Campos, A.; Giulianotti, M.A.; Castillo, R.; Medina-Franco, J.L. Structure-activity relationships of benzimidazole derivatives as antiparasitic agents: Dual activity-difference (DAD) maps. MedChemComm 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Pérez-Villanueva, J.; Hernández-Campos, A.; Castillo, R.; Meurice, N.; Medina-Franco, J.L. Chemoinformatic characterization of activity and selectivity switches of antiprotozoal compounds. Future Med. Chem. 2014, 6, 281–294. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villanueva, J.; Méndez-Lucio, O.; Soria-Arteche, O.; Medina-Franco, J.L. Activity cliffs and activity cliff generators based on chemotype-related activity landscapes. Mol. Divers. 2015, 19, 1021–1035. [Google Scholar] [CrossRef]
- Maggiora, G.M. On outliers and activity cliffs - Why QSAR often disappoints. J. Chem. Inf. Model. 2006, 46, 1535. [Google Scholar] [CrossRef]
- Bajorath, J.; Peltason, L.; Wawer, M.; Guha, R.; Lajiness, M.S.; Van Drie, J.H. Navigating structure–activity landscapes. Drug Discov. Today 2009, 14, 698–705. [Google Scholar] [CrossRef]
- Cruz-Monteagudo, M.; Medina-Franco, J.L.; Pérez-Castillo, Y.; Nicolotti, O.; Cordeiro, M.N.D.S.; Borges, F. Activity cliffs in drug discovery: Dr Jekyll or Mr Hyde? Drug Discov. Today 2014, 19, 1069–1080. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, C.; Larock, R.C.; Shi, F. Synthesis of 2H-indazoles by the [3 + 2] dipolar cycloaddition of sydnones with arynes. J. Org. Chem. 2011, 76, 8840–8851. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.R.; Park, A.; Park, N.; Lee, S. Consecutive condensation, C–N and N–N bond formations: A copper- catalyzed one-pot three-component synthesis of 2H-indazole. Org. Lett. 2011, 13, 3542–3545. [Google Scholar] [CrossRef]
- Genung, N.E.; Wei, L.; Aspnes, G.E. Regioselective Synthesis of 2H-indazoles using a mild, one-pot condensation–Cadogan reductive cyclization. Org. Lett. 2014, 16, 3114–3117. [Google Scholar] [CrossRef] [PubMed]
- Elie, J.; Vercouillie, J.; Arlicot, N.; Lemaire, L.; Bidault, R.; Bodard, S.; Hosselet, C.; Deloye, J.-B.; Chalon, S.; Emond, P.; et al. Design of selective COX-2 inhibitors in the (aza)indazole series. Chemistry, in vitro studies, radiochemistry and evaluations in rats of a [18F] PET tracer. J. Enzyme Inhib. Med. Chem. 2019, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Schoene, J.; Bel Abed, H.; Schmieder, P.; Christmann, M.; Nazaré, M. A general one-pot synthesis of 2H-indazoles using an organophosphorus–silane system. Chem. Eur. J. 2018, 24, 9090–9100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Z.; Peng, Q.; Zhou, Y.; Xu, L.; Pan, X. Access to 2-substituted-2H-indazoles via a copper-catalyzed regioselective cross-coupling reaction. Org. Biomol. Chem. 2018, 16, 1816–1822. [Google Scholar] [CrossRef]
- Nykaza, T.V.; Harrison, T.S.; Ghosh, A.; Putnik, R.A.; Radosevich, A.T. A biphilic phosphetane catalyzes N–N bond-forming Cadogan heterocyclization via PIII/PV=O redox cycling. J. Am. Chem. Soc. 2017, 139, 6839–6842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Medina, M.; Méndez-Lucio, O.; Medina-Franco, J.L. Activity landscape plotter: A web-based application for the analysis of structure–activity relationships. J. Chem. Inf. Model. 2017, 57, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Leland, B.A.; Henry, D.R.; Nourse, J.G. Reoptimization of MDL keys for use in drug discovery. J. Chem. Inf. Comput. Sci. 2002, 42, 1273–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 2010, 50, 742–754. [Google Scholar] [CrossRef] [PubMed]





 | |||
|---|---|---|---|
| Compound | R | Ultrasound Assisted One-Pot Procedure | Previously Reported by the Conventional Method [8] |
| 1 | H | 92 | 60 |
| 2 | 4-Cl | 62 | 52 |
| 3 | 4-OCH3 | 57 | 52 |
| 4 | 4-COOCH3 | 39 | 38 |
| 5 | 4-F | 71 | 50 |
| 6 | 4-CF3 | 43 | 6 |
| 9 | 3-Cl | 53 | - |
| 10 | 3-OCH3 | 45 | - |
| 11 | 3-COOCH3 | 51 | - |
| 12 | 3-F | 51 | - |
| 13 | 3-CF3 | 46 | - |
| 16 | 2-Cl | 55 | - |
| 17 | 2-OCH3 | 69 | - |
| 18a | 2-CN | 32 1 | - |
| 19 | 2-F | 54 | - |
| 20 | 2-CF3 | 26 | - |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R 1 | R 2 | R 3 | E. histolytica | G. intestinalis | T. vaginalis |
| 11 | H | H | H | 0.080 ± 0.004 | 0.113 ± 0.022 | 0.118 ± 0.022 |
| 21 | Cl | H | H | 0.042 ± 0.003 | 0.063 ± 0.003 | 0.107 ± 0.003 |
| 31 | OCH3 | H | H | 0.154 ± 0.016 | 0.205 ± 0.006 | 0.372 ± 0.016 |
| 41 | COOCH3 | H | H | 0.022 ± 0.003 | 0.063 ± 0.006 | 0.107 ± 0.006 |
| 5 | F | H | H | 0.075 ± 0.007 | 0.071 ± 0.007 | 0.118 ± 0.020 |
| 6 | CF3 | H | H | 0.053 ± 0.000 | 0.034 ± 0.005 | 0.084 ± 0.016 |
| 71 | OH | H | H | 0.074 ± 0.010 | 0.119 ± 0.007 | 0.157 ± 0.014 |
| 81 | COOH | H | H | 0.097 ± 0.006 | 0.193 ± 0.012 | 0.327 ± 0.018 |
| 9 | H | Cl | H | 0.070 ± 0.012 | 0.083 ± 0.012 | 0.101 ± 0.006 |
| 10 | H | OCH3 | H | 0.078 ± 0.010 | 0.056 ± 0.010 | 0.120 ± 0.019 |
| 11 | H | COOCH3 | H | 0.036 ± 0.000 | 0.054 ± 0.003 | 0.069 ± 0.008 |
| 12 | H | F | H | 0.097 ± 0.010 | 0.087 ± 0.003 | 0.092 ± 0.010 |
| 13 | H | CF3 | H | 0.061 ± 0.005 | 0.050 ± 0.005 | 0.069 ± 0.005 |
| 14 | H | OH | H | 0.102 ± 0.003 | 0.105 ± 0.000 | 0.162 ± 0.000 |
| 15 | H | COOH | H | 0.074 ± 0.009 | 0.053 ± 0.009 | 0.065 ± 0.009 |
| 16 | H | H | Cl | 0.088 ± 0.006 | 0.031 ± 0.006 | 0.066 ± 0.012 |
| 17 | H | H | OCH3 | 0.096 ± 0.003 | 0.083 ± 0.003 | 0.183 ± 0.006 |
| 18 | H | H | COOCH3 | 0.050 ± 0.003 | 0.032 ± 0.006 | 0.109 ± 0.008 |
| 19 | H | H | F | 0.092 ± 0.010 | 0.071 ± 0.007 | 0.108 ± 0.007 |
| 20 | H | H | CF3 | 0.031 ± 0.011 | 0.027 ± 0.011 | 0.128 ± 0.014 |
| 21 | H | H | OH | 0.145 ± 0.010 | 0.074 ± 0.003 | 0.112 ± 0.010 |
| 22 | H | H | COOH | 0.074 ± 0.003 | 0.023 ± 0.009 | 0.067 ± 0.006 |
| MTZ 2 | - | - | - | 0.380 ± 0.146 | 1.226 ± 0.125 | 0.236 ± 0.016 |
| ABZ 3 | - | - | - | 56.533 ± 18.845 | 0.037 ± 0.003 | 1.591 ± 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Villar, K.; Yépez-Mulia, L.; Cortés-Gines, M.; Aguilera-Perdomo, J.D.; Quintana-Salazar, E.A.; Olascoaga Del Angel, K.S.; Cortés-Benítez, F.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives. Molecules 2021, 26, 2145. https://doi.org/10.3390/molecules26082145
Rodríguez-Villar K, Yépez-Mulia L, Cortés-Gines M, Aguilera-Perdomo JD, Quintana-Salazar EA, Olascoaga Del Angel KS, Cortés-Benítez F, Palacios-Espinosa JF, Soria-Arteche O, Pérez-Villanueva J. Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives. Molecules. 2021; 26(8):2145. https://doi.org/10.3390/molecules26082145
Chicago/Turabian StyleRodríguez-Villar, Karen, Lilián Yépez-Mulia, Miguel Cortés-Gines, Jacobo David Aguilera-Perdomo, Edgar A. Quintana-Salazar, Kevin Samael Olascoaga Del Angel, Francisco Cortés-Benítez, Juan Francisco Palacios-Espinosa, Olivia Soria-Arteche, and Jaime Pérez-Villanueva. 2021. "Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives" Molecules 26, no. 8: 2145. https://doi.org/10.3390/molecules26082145
APA StyleRodríguez-Villar, K., Yépez-Mulia, L., Cortés-Gines, M., Aguilera-Perdomo, J. D., Quintana-Salazar, E. A., Olascoaga Del Angel, K. S., Cortés-Benítez, F., Palacios-Espinosa, J. F., Soria-Arteche, O., & Pérez-Villanueva, J. (2021). Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2H-Indazole Derivatives. Molecules, 26(8), 2145. https://doi.org/10.3390/molecules26082145