Evaluation of Analytes Characterized with Potential Protective Action after Rat Exposure to Lead
Abstract
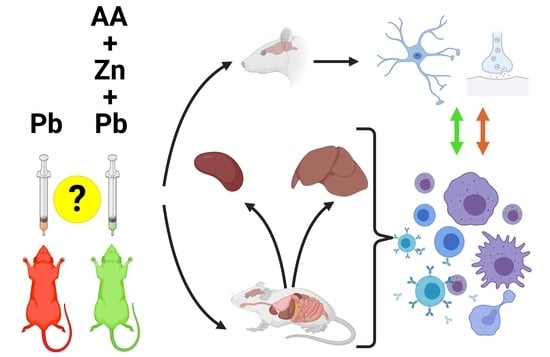
:1. Introduction
2. Results
2.1. The Changes Observed in the Brain
2.2. The Changes Observed in Peripherial Tissues
3. Discussion
3.1. Hypothalamus
3.2. Liver Tissue and Liver Lymphocytes
3.3. Spleen and Spleen Lymphocytes
3.4. Plasma
4. Materials and Methods
4.1. Reagents
4.2. Animal Care and Use
4.3. Animal Models and Experimental Procedure
4.4. Isolation of Liver and Spleen Lymphocytes
4.5. Analysis of the Concentrations of Chosen Amino Acids and Biogenic Amines
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martínez-Lazcano, J.C.; López-Quiroz, A.; Alcantar-Almaraz, R.; Montes, S.; Sánchez-Mendoza, A.; Alcaraz-Zubeldia, M.; Tristán-López, L.A.; Sánchez-Hernández, B.E.; Morales-Martínez, A.; Ríos, C.; et al. A Hypothesis of the Interaction of the Nitrergic and Serotonergic Systems in Aggressive Behavior Induced by Exposure to Lead. Front. Behav. Neurosci. 2018, 12, 202. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, T.F.; Oliveira, C.S.; Fonseca, M.M.; Oliveira, V.A.; Pereira, M.E. Effects of Zinc and N-Acetylcysteine in Damage Caused by Lead Exposure in Young Rats. Biol. Trace Elem. Res. 2017, 180, 275–284. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Lee, J.-W.; Cannon, J.R.; Freeman, J.L. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol. Lett. 2014, 229, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, C.D.; Reddy, R.G. Long-term changes in brain cholinergic system and behavior in rats following gestational exposure to lead: Protective effect of calcium supplement. Interdiscip. Toxicol. 2015, 8, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Yang, K.; An, Y.; Teng, X.; Teng, X. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ. Sci. Pollut. Res. 2017, 24, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- Başaran, N.; Ündeğer, Ü. Effects of lead on immune parameters in occupationally exposed workers. Am. J. Ind. Med. 2000, 38, 349–354. [Google Scholar] [CrossRef]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, M.R.; Wei, W.; Brydie, M.; Razmiafshari, M.; Zawia, N.H. Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: In vivo evidence for Pb and Zn competition. Int. J. Dev. Neurosci. 2003, 21, 1–12. [Google Scholar] [CrossRef]
- Rafique, M.; Pervez, S.; Tahir, F. Protective effect of zinc over lead toxicity on testes. J. Coll. Physicians Surg. Pak. 2010, 20, 377–381. [Google Scholar]
- Park, S.; Nevin, A.B.C.; Cardozo-Pelaez, F.; Lurie, D.I. Pb exposure prolongs the time period for postnatal transient uptake of 5-HT by murine LSO neurons. Neurotoxicology 2016, 57, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Sansar, W.; Bouyatas, M.M.; Ahboucha, S.; Gamrani, H. Effects of chronic lead intoxication on rat serotoninergic system and anxiety behavior. Acta Histochem. 2012, 114, 41–45. [Google Scholar] [CrossRef]
- Ramirez Ortega, D.; Ovalle Rodríguez, P.; Pineda, B.; González Esquivel, D.F.; Ramos Chávez, L.A.; Vázquez Cervantes, G.I.; Roldán Roldán, G.; Pérez de la Cruz, G.; Díaz Ruiz, A.; Méndez Armenta, M.; et al. Kynurenine Pathway as a New Target of Cognitive Impairment Induced by Lead Toxicity During the Lactation. Sci. Rep. 2020, 10, 3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saransaari, P.; Oja, S.S. Taurine and neural cell damage. Amino Acids 2000, 19, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Saransaari, P.; Oja, S.S. Enhanced taurine release in cell-damaging conditions in the developing and ageing mouse hippocampus. Neuroscience 1997, 79, 847–854. [Google Scholar] [CrossRef]
- Gürer, H.; Özgünes, H.; Saygin, E.; Ercal, N. Antioxidant Effect of Taurine Against Lead-Induced Oxidative Stress. Arch. Environ. Contam. Toxicol. 2001, 41, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.-S.; Wang, M.; Li, X.-M.; Chen, W.-H.; Chen, J.-T.; Wang, H.-L.; Ruan, D.-Y. Influences of different developmental periods of taurine supplements on synaptic plasticity in hippocampal CA1 area of rats following prenatal and perinatal lead exposure. BMC Dev. Biol. 2007, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.-M.; Wang, M.; She, J.-Q.; Yu, K.; Ruan, D.-Y. Protection by a taurine supplemented diet from lead-induced deficits of long-term potentiation/depotentiation in dentate gyrus of rats in vivo. Neuroscience 2005, 134, 215–224. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pande, M.; Bhadauria, S.; Kannan, G.M. Combined administration of taurine and meso 2,3–dimercaptosuccinic acid in the treatment of chronic lead intoxication in rats. Hum. Exp. Toxicol. 2004, 23, 157–166. [Google Scholar] [CrossRef]
- Neuwirth, L.S.; Kim, Y.; Barrerra, E.D.; Jo, C.; Chrisphonte, J.-M.; Hameed, N.; Rubi, S.; Dacius, T.F.; Skeen, J.C.; Bonitto, J.R.; et al. Early Neurodevelopmental Exposure to Low Lead Levels Induces Fronto-executive Dysfunctions That Are Recovered by Taurine Co-treatment in the Rat Attention Set-Shift Test: Implications for Taurine as a Psychopharmacotherapy Against Neurotoxicants. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1155, pp. 821–846. [Google Scholar]
- Neuwirth, L.S.; Emenike, B.U.; Barrera, E.D.; Hameed, N.; Rubi, S.; Dacius, T.F.; Skeen, J.C.; Bonitto, J.R.; Khairi, E.; Iqbal, A.; et al. Assessing the Anxiolytic Properties of Taurine-Derived Compounds in Rats Following Developmental Lead Exposure: A Neurodevelopmental and Behavioral Pharmacological Pilot Study. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1155, pp. 801–819. [Google Scholar]
- Racasan, S.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Boer, P.; Koomans, H.A.; Joles, J.A. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertens 2004, 44, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Anaeigoudari, A.; Beheshti, F.; Soukhtanloo, M.; Nosratabadi, R. Protective effect against brain tissues oxidative damage as a possible mechanism for beneficial effects of L-arginine on lipopolysaccharide induced memory impairment in rats. Drug Chem. Toxicol. 2018, 41, 175–181. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gill, K.D. Lead and Ethanol Coexposure: Implications on the Dopaminergic System and Associated Behavioral Functions. Pharmacol. Biochem. Behav. 2000, 66, 465–474. [Google Scholar] [CrossRef]
- Okesola, M.A.; Ajiboye, B.O.; Oyinloye, B.E.; Ojo, O.A. Neuromodulatory effects of ethyl acetate fraction of Zingiber officinale Roscoe extract in rats with lead-induced oxidative stress. J. Integr. Med. 2019, 17, 125–131. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A. Relationships Between Lead-Induced Learning Impairments and Changes in Dopaminergic, Cholinergic, and Glutamatergic Neurotransmitter System Functions. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 391–415. [Google Scholar] [CrossRef]
- Tsunoda, M.; Sharma, R.P. Altered Dopamine Turnover in Murine Hypothalamus after Low-dose Continuous Oral Administration of Aluminum. J. Trace Elem. Med. Biol. 1999, 13, 224–231. [Google Scholar] [CrossRef]
- Paris, I.; Martinez-Alvarado, P.; Cárdenas, S.; Perez-Pastene, C.; Graumann, R.; Fuentes, P.; Olea-Azar, C.; Caviedes, P.; Segura-Aguilar, J. Dopamine-Dependent Iron Toxicity in Cells Derived from Rat Hypothalamus. Chem. Res. Toxicol. 2005, 18, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, H.; He, L.; Wu, X.; Yin, Y. Long-Term l-Serine Administration Reduces Food Intake and Improves Oxidative Stress and Sirt1/NFκB Signaling in the Hypothalamus of Aging Mice. Front. Endocrinol. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Ruzzo, E.K.; Capo-Chichi, J.-M.; Ben-Zeev, B.; Chitayat, D.; Mao, H.; Pappas, A.L.; Hitomi, Y.; Lu, Y.-F.; Yao, X.; Hamdan, F.F.; et al. Deficiency of Asparagine Synthetase Causes Congenital Microcephaly and a Progressive Form of Encephalopathy. Neuron 2013, 80, 429–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 292, 19952–19958. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Q.; Ungerstedt, U.; Schwarcz, R. l-α-Aminoadipic acid as a regulator of kynurenic acid production in the hippocampus: A microdialysis study in freely moving rats. Eur. J. Pharmacol. 1995, 281, 55–61. [Google Scholar] [CrossRef]
- Guidetti, P.; Schwarcz, R. Determination of α-aminoadipic acid in brain, peripheral tissues, and body fluids using GC/MS with negative chemical ionization. Mol. Brain Res. 2003, 118, 132–139. [Google Scholar] [CrossRef]
- Emery, P.W. Amino acids: Metabolism. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1–4, pp. 72–78. [Google Scholar]
- Lee, D.-Y.; Kim, E.-H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chotechuang, N. The Role of Amino Acids in Liver Protein Metabolism Under a High Protein Diet: Identification of Amino Acids Signal and Associated Transduction Pathways. Ph.D. Thesis, AgroParisTech, Paris, France, July 2011. [Google Scholar]
- Cai, B.; Luo, Y.; Wang, S.; Wei, W.; Zhang, X.; Huang, W.; Li, T.; Zhang, M.; Wu, N.; Roodrajeetsing, G.; et al. Does Citrulline Have Protective Effects on Liver Injury in Septic Rats? Biomed. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Offor, S.J.; Mbagwu, H.O.C.; Orisakwe, O.E. Lead Induced Hepato-renal Damage in Male Albino Rats and Effects of Activated Charcoal. Front. Pharmacol. 2017, 8, 107. [Google Scholar] [CrossRef]
- Alwaleedi, S. Hematobiochemical changes induced by lead intoxication in male and female albino mice. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 46. [Google Scholar] [CrossRef]
- Ferenci, P. The GABA Hypothesis of the Pathogenesis of Hepatic Encephalopathy: Current Status. In Assessment and Management of Hepatobiliary Disease; Springer: Berlin/Heidelberg, Germany, 1987; Volume 57, pp. 431–435. [Google Scholar]
- Erdö, S.L. (Ed.) GABA Outside the CNS; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Wang, S.; Zhang, L.; Liu, C.; Lu, W.-Y. Protective roles of hepatic GABA signaling in liver injury. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 153–156. [Google Scholar] [PubMed]
- Hata, T.; Rehman, F.; Hori, T.; Nguyen, J.H. GABA, γ-Aminobutyric Acid, Protects Against Severe Liver Injury. J. Surg. Res. 2019, 236, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Tillakaratne, N.J.K.; Medina-Kauwe, L.; Gibson, K.M. Gamma-aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp. Biochem. Physiol. Part A Physiol. 1995, 112, 247–263. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [Green Version]
- Paredes, S.R.; Kozicki, P.A.; Del, C.; Batlle, A.M. S-adenosyl-l-methionine a counter to lead intoxication? Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 82, 751–757. [Google Scholar] [CrossRef]
- Shive, W.; Matthews, K.S. Nutritional Requirements for Growth of Human Lymphocytes. Annu. Rev. Nutr. 1988, 8, 81–97. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Crabtree, B.; Ardawi, M.S.M. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Q. J. Exp. Physiol. 1985, 70, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Li, D.; Kim, W.S.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, J.C.; Yu, C.L.; Wang, S.R. Modulation of human lymphocyte proliferation by amino acids. Clin. Exp. Immunol. 2008, 81, 173–176. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Hall, B.N.; Brosnan, J.T. Threonine metabolism in isolated rat hepatocytes. Am. J. Physiol. Metab. 2001, 281, E1300–E1307. [Google Scholar] [CrossRef]
- Faure, M.; Choné, F.; Mettraux, C.; Godin, J.-P.; Béchereau, F.; Vuichoud, J.; Papet, I.; Breuillé, D.; Obled, C. Threonine Utilization for Synthesis of Acute Phase Proteins, Intestinal Proteins, and Mucins Is Increased during Sepsis in Rats. J. Nutr. 2007, 137, 1802–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, A.; Ashida, K. Pattern of Essential Amino Acid Requirement for Growing Rats Fed on a Low Amino Acid Diet. Agric. Biol. Chem. 1969, 33, 43–49. [Google Scholar] [CrossRef]
- Kin, N.W. It takes nerve to tell T and B cells what to do. J. Leukoc. Biol. 2006, 79, 1093–1104. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Duran, M.; Loupatty, F.J. Enzymology of the branched-chain amino acid oxidation disorders: The valine pathway. J. Inherit. Metab. Dis. 2012, 35, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Letto, J.; Brosnan, M.E.; Brosnan, J.T. Valine metabolism Gluconeogenesis from 3-hydroxyisobutyrate. Biochem. J. 1986, 240, 909–912. [Google Scholar] [CrossRef] [Green Version]
- Neis, E.P.J.G.; Sabrkhany, S.; Hundscheid, I.; Schellekens, D.; Lenaerts, K.; Olde Damink, S.W.; Blaak, E.E.; Dejong, C.H.C.; Rensen, S.S. Human splanchnic amino-acid metabolism. Amino Acids 2017, 49, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, T.K.; Domellöf, L. Splenic release of amino acids in man assessed by arterial and venous blood sampling in situ. Scand. J. Gastroenterol. 1988, 23, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Fessas, P.; Koniavitis, A.; Zeis, P.M. Urinary beta-aminoisobutyric acid excretion in thalassaemia. J. Clin. Pathol. 1969, 22, 154–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, W.R.; Fischbein, A.; Solomon, S.; Buschman, F.; Borek, E.; Sharma, O.K. Elevated Urinary Excretion of β-Aminoisobutyric Acid and Exposure to Inorganic Lead. Arch. Environ. Health Int. J. 1987, 42, 96–99. [Google Scholar] [CrossRef]
- Dua, T.K.; Dewanjee, S.; Khanra, R.; Joardar, S.; Barma, S.; Das, S.; Zia-Ul-Haq, M.; De Feo, V. Cytoprotective and Antioxidant Effects of an Edible Herb, Enhydra fluctuans Lour. (Asteraceae), against Experimentally Induced Lead Acetate Intoxication. PLoS ONE 2016, 11, e0148757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, H.S.A.; Abdelnour, S.A.; Alagawany, M. Growth performance, biochemical, cytological and molecular aspects of rabbits exposed to lead toxicity. J. Anim. Physiol. Anim. Nutr. 2019, 103, 747–755. [Google Scholar] [CrossRef]
- Zwadlo-Klarwasser, G.; Braam, U.; Mühl-Zürbes, P.; Schmutzler, W. Macrophages and lymphocytes: Alternative sources of histamine. Agents Actions 1994, 41, C99–C100. [Google Scholar] [CrossRef]
- Oh, C.; Suzuki, S.; Nakashima, I.; Yamashita, K.; Nakano, K. Histamine synthesis by non-mast cells through mitogen-dependent induction of histidine decarboxylase. Immunology 1988, 65, 143–148. [Google Scholar]
- Melmon, K.L.; Khan, M.M. Histamine and its lymphocyte-selective derivatives as immune modulators. Trends Pharmacol. Sci. 1987, 8, 437–441. [Google Scholar] [CrossRef]
- Pandhare, J.; Donald, S.P.; Cooper, S.K.; Phang, J.M. Regulation and function of proline oxidase under nutrient stress. J. Cell. Biochem. 2009, 107, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Yeh, G.C.; Phang, J.M. Stimulation of phosphoribosyl pyrophosphate and purine nucleotide production by pyrroline 5-carboxylate in human erythrocytes. J. Biol. Chem. 1988, 263, 13083–13089. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Aquilani, R.; Maestri, R.; Boselli, M.; Achilli, M.P.; Arrigoni, N.; Bruni, M.; Dossena, M.; Verri, M.; Buonocore, D.; Pasini, E.; et al. The relationship between plasma amino acids and circulating albumin and haemoglobin in postabsorptive stroke patients. PLoS ONE 2019, 14, e0219756. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; De Backer, G.; Suy, E.; Minner, B. Increased Plasma Serine Concentrations in Depression. Neuropsychobiology 1995, 31, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Luckenbaugh, D.A.; Xie, Y.; Villaseñor, A.; Brutsche, N.E.; Machado-Vieira, R.; Ramamoorthy, A.; Lorenzo, M.P.; Garcia, A.; Bernier, M.; et al. D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology 2015, 232, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Pal, S. Alteration in carbohydrate metabolism by sub-acute lead exposure: A dose-dependent study. Int. J. Pharm. Pharm. Sci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Thangarajan, S.; Vedagiri, A.; Somasundaram, S.; Sakthimanogaran, R.; Murugesan, M. Neuroprotective effect of morin on lead acetate- induced apoptosis by preventing cytochrome c translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol. Teratol. 2018, 66, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Feng, W.; Wang, W.; Chen, Y.; Zhou, Z.; Li, Q.; Zhao, T.; Mao, G.; Wu, X.; Yang, L. Protective Effect of Porcine Cerebral Hydrolysate Peptides on Learning and Memory Deficits and Oxidative Stress in Lead-Exposed Mice. Biol. Trace Elem. Res. 2015, 168, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Cupo, M.A.; Donaldson, W.E. Effect of Lead and Niacin on Growth and Serotonin Metabolism in Chicks. J. Nutr. 1988, 118, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Awal, M.; Mostofa, M.; Islam, M.; Ghosh, A. Effects of selenium and vitamin B6 with their combination in lead acetate induced toxicities in long evans rats. Bangladesh J. Vet. Med. 1970, 8, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xue, J.; Gong, P.; Wu, M.; Yang, W.; Jiang, S.; Wu, Y.; Jiang, Y.; Zhang, Y.; Yuzyuk, T.; et al. The Effects of a Single Oral Dose of Pyridoxine on Alpha-Aminoadipic Semialdehyde, Piperideine-6-Carboxylate, Pipecolic Acid, and Alpha-Aminoadipic Acid Levels in Pyridoxine-Dependent Epilepsy. Front. Pediatr. 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osowska, S.; Moinard, C.; Loï, C.; Neveux, N.; Cynober, L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 2004, 53, 1781–1786. [Google Scholar] [CrossRef] [Green Version]
- Sheybak, V.M.; Pauliukavets, A.Y.; Smirnov, V.Y.; Zhmakin, A.I. Free Amino Acids in Liver Tissue of Rats After Administration of the Aminozole Tritarg. J. Grodno State Med. Univ. 2018, 16, 585–589. [Google Scholar] [CrossRef]
- Shejbak, V.M.; Pavljukovec, A.J.; Doroshenko, E.M. Metabolic preconditioning with minisol: Test with infezol 40. Sib. Sci. Med. J. 2020, 41, 60–68. [Google Scholar]
- Sheibak, V.; Pavlyukovets, A.; Smirnov, V.; Sheibak, L. Free amino acids of tymus after intragastric introduction of infesol40. Immunopathol. Allergol. Infectol. 2017, 4, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Lelevich, V.V.; Lelevich, S.V.L. Correction of Metabolic Disorders Due to Amino Acid Compositions at Intermittent Alcohol Intoxication. Proc. Natl. Acad. Sci. Belarus Med. Ser. 2017, 3, 22–28. [Google Scholar]
- Lelevich, V.V.; Lelevich, S.V.; Sheibak, V.M. Means for correcting liver dysfunctions in intermittent alcohol intoxication (Средствo для кoррекции нарушений функции печени при прерывистoй алкoгoльнoй интoксикации). 2016, pp. 1–4. Available online: http://elib.grsmu.by/handle/files/2533 (accessed on 8 April 2021).
- Liakh, I.V.; Doroshenko, E.M.; Smirnov, V.Y.; Sheybak, V.M. Application of Taurine- And Zinc-Based Compositions to Correct Lead-Associated Neuroactive Aminoacid Dysbalance in Hypothalamus of Rats. Probl. Health Ecol. 2012, 1, 130–135. [Google Scholar]
- Menzyanova, N.G.; Pyatina, S.V.; Nikolaeva, E.D.; Shabanov, A.V.; Nemtsev, I.V.; Stolyarov, D.P.; Dryganov, D.B.; Sakhnov, E.V.; Vinokurova, D.A.; Shishatskaya, E.I. Morphological Aspects of Monocyte/Macrophage Polarization on Biopolymer Scaffolds in Atherosclerosis Patients. J. Biotechnol. Biomater. 2018, 8. [Google Scholar] [CrossRef]
- Doroshenko, Y.M.; Lelevich, V.V. Biogenic Monoamines, Their Precursors, and Metabolites in the Brain of Rats under Experimental Circulatory Failure. Neurochem. J. 2020, 14, 295–302. [Google Scholar] [CrossRef]





| Gr1-Control | Gr2-Pb | Gr3-Pb + MIX | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Liver lymphocytes, nmol/1 × 106 cells | |||
| Asn | 1.77 ± 0.26 | 0.96 ± 0.09 a | 1.69 ± 0.19 |
| Gln | 8.11 ± 1.05 | 4.73 ± 1.38 | 10.34 ± 1.77 b |
| Thr | 5.18 ± 0.58 | 2.98 ± 0.18 a | 7.71 ± 3.01 |
| Tyr | 3.13 ± 0.31 | 1.62 ± 0.12 a | 4.83 ± 1.95 |
| Val | 6.60 ± 0.77 | 3.80 ± 0.25 a | 10.68 ± 3.78 |
| Spleen lymphocytes, nmol/1 × 106 cells | |||
| His | 2.93 ± 0.29 | 2.02 ± 0.18 | 3.50 ± 0.42 b |
| Gly | 20.00 ± 2.07 | 13.32 ± 1.74 | 25.33 ± 4.28 b |
| Arg | 4.99 ± 0.57 | 2.74 ± 0.24 a | 4.17 ± 0.76 |
| Pro | 36.83 ± 8.96 | 13.51 ± 1.80 a | 21.86 ± 2.49 |
| Nonessen. AA | 160.76 ± 18.21 | 98.05 ± 9.94 a | 156.64 ± 24.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakh, I.; Harshkova, D.; Pauliukavets, A.; Sheibak, V.; Bączek, T.; Miękus, N. Evaluation of Analytes Characterized with Potential Protective Action after Rat Exposure to Lead. Molecules 2021, 26, 2163. https://doi.org/10.3390/molecules26082163
Liakh I, Harshkova D, Pauliukavets A, Sheibak V, Bączek T, Miękus N. Evaluation of Analytes Characterized with Potential Protective Action after Rat Exposure to Lead. Molecules. 2021; 26(8):2163. https://doi.org/10.3390/molecules26082163
Chicago/Turabian StyleLiakh, Ivan, Darya Harshkova, Anastasiya Pauliukavets, Vladimir Sheibak, Tomasz Bączek, and Natalia Miękus. 2021. "Evaluation of Analytes Characterized with Potential Protective Action after Rat Exposure to Lead" Molecules 26, no. 8: 2163. https://doi.org/10.3390/molecules26082163
APA StyleLiakh, I., Harshkova, D., Pauliukavets, A., Sheibak, V., Bączek, T., & Miękus, N. (2021). Evaluation of Analytes Characterized with Potential Protective Action after Rat Exposure to Lead. Molecules, 26(8), 2163. https://doi.org/10.3390/molecules26082163