Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nematostatic and Nematicidal Effect and EC50 of Extracts
2.2. Compounds Identified in A. aurantium and their Nematostatic Effects
2.3. Compounds Identified in A. integrifolium
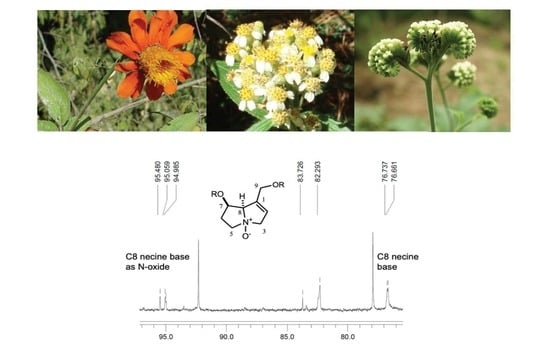
2.4. Compounds Identified in T. densiflora
2.5. Phytotoxicity Test
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemicals
3.3. Plant Species
3.4. Preparation of Extracts
3.5. Isolation of 1–3 from A. aurantium Extract
3.6. Identification of Compounds from A. integrifolium
3.7. Identification of Compounds from T. densiflora Roots
3.8. Screening of Nematicidal and Nematostatic Activities
3.8.1. Nematodes
3.8.2. Assay
3.9. Phytotoxicity Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Flores-Camacho, R.; Manzanilla-López, R.H.; Cid del Prado-Vera, I.; Martínez-Garza, A. Control de Nacobbus aberrans (Thorne) Thorne y Allen con Pochonia chlamydosporia (Goddard) Gams y Zare. Rev. Mex. Fitopatol. 2007, 25, 26–34. [Google Scholar]
- Pérez-Rodríguez, I.; Franco-Navarro, F.; Cid del Prado-Vera, I.; Zavaleta-Mejía, E. Control de Nacobbus aberrans en chile ancho (Capsicum annuum L.) mediante el uso combinado de enmiendas orgánicas, hongos nematófagos y nematicidas. Nematropica 2011, 41, 122–129. [Google Scholar]
- Lax, P.; Marro, N.; Agaras, B.; Valverde, C.; Doucet, M.E.; Becerra, A. Biological control of the false root-knot nematode Nacobbus aberrans by Pseudomonas protegens under controlled conditions. Crop Prot. 2013, 52, 97–102. [Google Scholar] [CrossRef]
- Caccia, M.; Lax, P.; Doucet, M.E. Effect of entomopathogenic nematodes on the plants-parasitic nematode Nacobbus aberrans. Biol. Fertil. Soils 2013, 49, 105–109. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, M.; Medina-Medrano, J.R.; Cortez-Madrigal, H.; Angoa-Pérez, M.V.; Muñoz-Ruíz, C.V.; Villar-Luna, E. Nematicidal activity of wild plant extracts against second-stage juveniles of Nacobbus aberrans. Nematropica 2018, 48, 136–144. [Google Scholar]
- Ntalli, N.; Kasiotis, K.M.; Baira, E.; Stamatis, C.L.; Machera, K. Nematicidal activity of Stevia rebaudiana (Bertoni) assisted by phytochemical analysis. Toxins 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Ozalexandridou, E.X.; Kasiotis, K.M.; Samara, M.; Golfinopoulos, S.K. Nematicidal activity and phytochemistry of Greek Lamiaceae species. Agronomy 2020, 10, 1119. [Google Scholar] [CrossRef]
- Zavaleta-Mejía, E.; Gómez, R.O. Effect of Tagetes erecta L.-Tomato (Lycopersicon esculentum Mill.) intercropping on some tomato pests. Fitopatología 1995, 30, 33–45. [Google Scholar]
- Mareggiani, G.; Zamuner, N.; Michetti, M.; Franzetti, D.; Collavino, M. Impact of natural extracts on target and non-target soil organisms. Bol. San. Veg. Plagas 2005, 31, 443–448. [Google Scholar]
- Godínez-Vidal, D.; Soto-Hernández, M.; Rocha-Sosa, M.; Lozoya-Gloria, E.; Rojas-Martínez, R.I.; Guevara-Olvera, L.; Zavaleta-Mejía, E. Contenido de capsidiol en raíces de chile CM-334 infectadas por Nacobbus aberrans y su efecto en juveniles del segundo estadio. Nematropica 2010, 40, 227–237. [Google Scholar]
- Rodríguez-Chávez, J.L.; Franco-Navarro, F.; Delgado, G. In vitro nematicidal activity of natural and semisynthetic cadinenes from Heterotheca inuloides against the plant-parasitic nematode Nacobbus aberrans (Tylenchida: Pratylenchidae). Pest Manag. Sci. 2019, 75, 1734–1742. [Google Scholar] [CrossRef]
- Cristóbal-Alejo, J.; Mora-Aguilera, G.; Manzanilla-Lopez, R.H.; Marbán-Méndoza, N.; Sánchez-Garcia, P.; Del Prado-Vera, I.C.; Evans, K. Epidemiology and integrated control of Nacobbus aberrans on tomato in Mexico. Nematology 2006, 8, 727–737. [Google Scholar] [CrossRef]
- Feist, E.; Kearn, J.; Gaihre, Y.; O’Connor, V.; Holden-Dye, L. The distinct profiles of the inhibitory effects of fluensulfone, abamectin, aldicarb and fluopyram on Globodera pallida hatching. Pestic. Biochem. Physiol. 2020, 165, 104541. [Google Scholar] [CrossRef]
- Forgo, P.; Kövér, K.E. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. SpringerPlus 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Martínez, M.; Hernández-Ramírez, V.I.; Hernández-Carlos, B.; Chávez-Munguía, B.; Calderón-Oropeza, M.A.; Talamás-Rohana, P. Antiamoebic activity of Adenophyllum aurantium (L.) Strother and its effect on the actin cytoskeleton of Entamoeba histolytica. Front. Pharmacol. 2016, 7, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downum, K.R.; Keil, D.J.; Rodríguez, E. Distribution of acetylenic thiophenes in the pectidinae. Biochem. Syst. Ecol. 1985, 13, 109–113. [Google Scholar] [CrossRef]
- Marotti, I.; Marotti, M.; Piccaglia, R.; Nastri, A.; Grandi, S.; Dinelli, G. Thiophene occurrence in different Tagetes species: Agricultural biomasses as sources of biocidal substances. J. Sci. Food Agric. 2010, 90, 1210–1217. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Wang, K.H.; Ploeg, A.; McSorley, R. Using marigold (Tagetes spp.) as a cover crop to protect crops from plant-parasitic nematodes. Appl. Soil Ecol. 2010, 46, 307–320. [Google Scholar] [CrossRef]
- Kimura, Y.; Hiraoka, K.; Kawano, T.; Fujioka, S.; Shimada, A. Nematicidal activities of acetylene compounds from Coriopsis lanceolata L. Zeitschrift für Naturforschung C 2008, 63, 843–847. [Google Scholar] [CrossRef]
- Faizi, S.; Fayyaz, S.; Bano, S.; Yawar Iqbal, E.; Lubna, L.; Siddiqi, H.; Naz, A. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: Structure-activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef] [PubMed]
- Ferheen, S.; Akhtar, M.; Ahmed, A.G.; Anwar, M.A.; Kalhoro, M.A.; Afza, N.; Malik, A. Nematicidal potential of the Galinsoga parviflora. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2011, 54, 83–87. [Google Scholar]
- Barbosa, L.C.A.; Barcelos, F.F.; Demuner, A.J.; Santos, M.A. Chemical constituents from Mucuma aterrima with activity against Meloidogyne incognita and Heterodera glycines. Nematropica 1999, 29, 81–88. [Google Scholar]
- Naz, I.; Khan, M.R. Nematicidal activity of nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol isolated from the roots of Fumaria parviflora (Fumariaceae). J. Agric. Food Chem. 2013, 61, 5689–5695. [Google Scholar] [CrossRef]
- Naz, I.; Saifullah, S.; Palomares-Rius, J.E.; Ahmad, M.; Ali, A.; Rashid, M.U.; Bibi, F. Combined nematocidal effect of nonacosan-10-ol and 23a-homostigmast-5-en-3β-ol on Meloidogyne incognita (kofoid and white) chitwood. J. Anim. Plant Sci. 2016, 26, 1633–1640. [Google Scholar]
- Chitwood, D.J.; McClure, M.A.; Feldlaufer, M.F.; Lusby, W.R.; Oliver, T.E. Sterol composition and ecdysteroid content of eggs of the root-knot nematodes Meloidogyne incognita and M. arenaria. J. Nematol. 1987, 19, 352. [Google Scholar]
- Udalova, Z.V.; Zinov’eva, S.V.; Valis’eva, I.S.; Paseshnichenko, V.A. Correlation between the structure of plant steroids and their effects on phytoparasitic nematodes. Appl. Biochem. Microbiol. 2004, 40, 93–97. [Google Scholar] [CrossRef]
- Moss, G.P. Carbon-13 NMR spectra of carotenoids. Pure Appl. Chem. 1976, 47, 97–102. [Google Scholar] [CrossRef] [Green Version]
- El-Raey, M.A.; Ibrahim, G.E.; Eldahshan, O.A. Lycopene and lutein; A review for their chemistry and medicinal uses. J. Pharmacogn. Phytochem. 2013, 2, 245–254. [Google Scholar]
- Chiang, Y.M.; Chuang, D.Y.; Wang, S.Y.; Kuo, Y.H.; Tsai, P.W.; Shyur, L.F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Tapia, A.; Theoduloz, C.; Rodríguez, J.; López, S.; Feresin, G.E. Free radical scavengers and antioxidants from Tagetes mendocina. Zeitschrift für Naturforschung C 2004, 59, 345–353. [Google Scholar] [CrossRef]
- Arisawa, M.; Hatashita, T.; Numata, Y.; Tanaka, M.; Sasaki, T. Cytotoxic principles from Chrysosplenium flagelliferum. Int. J. Pharmacogn. 1997, 35, 141–143. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Zhou, Z.; Lai, C.S.; Zhang, L.; Quan, Z.; Shao, X.; Pan, M.-H.; Ho, C.T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010, 122, 831–835. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. C17-Acetylenverbindungen aus Calea integrifolia. Phytochemistry 1976, 15, 1177. [Google Scholar] [CrossRef]
- Lira-De León, K.I.; Herrera-Martínez, M.; Ramírez-Mares, M.V.; Hernández-Carlos, B. Evaluation of anticancer potential of eight vegetal species from the state of Oaxaca. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, T.C.; de Jesus Souza, R.; da Silva, F.A.; Biavatti, M.W. The genus Calea L.: A review on traditional uses, phytochemistry and biology activities. Phytother. Res. 2018, 32, 769–795. [Google Scholar] [CrossRef]
- Bano, S.; Iqbal, E.Y.; Lubna; Zil-ur-Rehman, S.; Fayyaz, S.; Faizi, S. Nematicidal activity of flavonoids with structure activity relationship (SAR) studies against root knot nematode Meloidogyne incognita. Eur. J. Plant Pathol. 2020, 157, 299–309. [Google Scholar] [CrossRef]
- Isaac, R.E.; MacGregor, D.; Coates, D. Metabolism and inactivation of neurotransmitters in nematodes. Parasitology 1996, 113, S157–S173. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Zeitschrift für Naturforschung C 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Promchai, T.; Saesong, T.; Ingkaninan, K.; Laphookhieo, S.; Pyne, S.G.; Limtharakul, T. Acetylcholinesterase inhibitory activity of chemical constituents isolated from Miliusa thorelii. Phytochem. Lett. 2018, 23, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gao, X.; Lan, M.; Liao, X.; Su, F.; Fan, L.; Zhao, Y.; Hao, X.; Wu, G.; Ding, X. Inhibitory activities of flavonoids from Eupatorium adenophorum against acetylcholinesterase. Pestic. Biochem. Physiol. 2020, 170, 104701. [Google Scholar] [CrossRef] [PubMed]
- San Martín, R.; Magunacelaya, J.C. Control of plant-parasitic nematodes with extracts of Quillaja saponaria. Nematology 2005, 7, 577–585. [Google Scholar]
- Correia Da Silva, T.B.; Souza, V.K.T.; Da Silva, A.P.F.; Lyra-Lemos, R.P.; Conserva, L.M. Determination of the phenolic content and antioxidant potential of crude extracts and isolated compounds from leaves of Cordia multispicata and Tournefortia bicolor. Pharm. Biol. 2010, 48, 63–69. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, K.; Miyagi, Y.; Higa, M.; Yogi, S. Pyrrolizidine alkaloids from Messerschmidia argentea. Phytochemistry 1997, 44, 545–547. [Google Scholar] [CrossRef]
- Constantinidis, T.; Harvala, C.; Skaltsounis, A.L. Pyrrolizidine N-oxide alkaloids of Heliotropium hirsutissimum. Phytochemistry 1993, 32, 1335–1337. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Roitman, J.N.; Benson, M.; Lundin, R.E. 13C NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1982, 21, 439–443. [Google Scholar] [CrossRef]
- Hammouda, F.M.; Ismail, S.I.; Hassan, N.M.; Tawfiq, W.A.; Kamel, A. Pyrrolizidine alkaloids from Alkanna orientalis (L.) Boiss. Qatar Univ. Sci. J. 1992, 12, 80–82. [Google Scholar]
- Logie, C.G.; Grue, M.R.; Liddell, J.R. Proton NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1994, 37, 43–109. [Google Scholar] [CrossRef]
- Roeder, E. Carbon-13 NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1990, 29, 11–29. [Google Scholar] [CrossRef]
- Lira-De León, K.I.; Ramírez-Mares, M.V.; Sánchez-López, V.; Ramírez Lepe, M.; Salas-Coronado, R.; Santos-Sánchez, N.F.; Valadez-Blanco, R.; Hernández-Carlos, B. Effect of crude plant extracts from some Oaxacan flora on two deleterious fungal phytopathogens and extracts compatibility with a biofertilizer strain. Front. Microbiol. 2014, 5, 383. [Google Scholar] [CrossRef]
- van Dam, N.M.; Vuister, L.W.; Bergshoeff, C.; de Vos, H.; Van der Meijden, E.D. The “Raison D’être” of pyrrolizidine alkaloids in Cynoglossum officinale: Deterrent effects against generalist herbivores. J. Chem. Ecol. 1995, 21, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Thoden, T.C.; Boppré, M.; Hallmann, J. Effects of pyrrolizidine alkaloids on the performance of plant-parasitic and free-living nematodes. Pest Manag. Sci. 2009, 65, 823–830. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, D.J.; Lusby, W.R. Metabolism of plants sterol by Nematodes. Lipids 1991, 26, 619–626. [Google Scholar] [CrossRef]
- Nivsarkar, M.; Kumar, G.P.; Laloraya, M.; Laloraya, M.M. Superoxide dismutase in the anal gills of the mosquito larvae of Aedes aegypti: Its inhibition by α-terthienyl. Arch. Insect Biochem. Physiol. 1991, 16, 249–255. [Google Scholar] [CrossRef]
- MacRae, W.D.; Chan, G.F.Q.; Wat, C.K.; Towers, G.H.N.; Lam, J. Examination of naturally occurring polyacetylenes and α-terthienyl for their ability to induce cytogenetic damage. Experientia 1980, 36, 1096–1097. [Google Scholar] [CrossRef]
- Sripathi, S.K.; Gopal, P.; Lalitha, P. Allantoin from the leaves of Pisonia grandis R. Br. Int. J. Pharm. Life Sci. 2011, 2, 815–817. [Google Scholar]
- Argentieri, M.P.; D’Addabbo, T.; Tava, A.; Agostinelli, A.; Jurzysta, M.; Avato, P. Evaluation of nematicidal properties of saponins from Medicago spp. Eur. J. Plant Pathol. 2008, 120, 189–197. [Google Scholar] [CrossRef]
- Hernández-Carlos, B.; González-Coloma, A.; Orozco-Valencia, A.U.; Ramírez-Mares, M.V.; Andrés-Yeves, M.F.; Joseph-Nathan, P. Bioactive saponins from Microsechium helleri and Sycios bulbosus. Phytochemistry 2011, 72, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Extract | % Immobility J2s | ||||||
|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 72 h * | |
| A. aurantium A | 84.6 ± 6 a | 89.32 ± 1 b | 97.0 ± 2 bc | 98.1 ± 3 bc | 99.3 ± 1.1 c | 99.2 ± 1.5 c | 96.4 ± 5.4 bc |
| A. aurantium R | 61.0 ± 16 a | 65.97 ± 6 a | 95.3 ± 4 b | 99.0 ± 0 b | 100 ± 0 b | 100 ± 0.0 b | 99.8 ± 0.5 b |
| A. cuspidata | 82.3 ± 5 a | 86.6 ± 4 a | 94.6 ± 3 ab | 96.0 ± 1 b | 98.3 ± 2 b | 98.9 ± 2 b | 90.6 ± 5 a |
| A. integrifolium | 61.8 ± 13 a | 94.1 ± 3 b | 93.3 ± 4 b | 96.5 ± 2 b | 99.6 ± 1 b | 99.8 ± 0.4 b | 99.4 ± 1 b |
| A. subviscida | 78.9 ± 10 a | 79.3 ± 10 a | 91.3 ± 6 b | 93.5 ± 6 b | 98.1 ± 2 b | 99.3 ± 1 b | 95.4 ± 2 b |
| G. mexicanum | 62.9 ± 23 a | 85.3 ± 10 b | 93.7 ± 3 b | 96.2 ± 3 b | 98.5 ± 2 b | 98.6 ± 2 b | 94.2 ± 3 b |
| H. terebinthinaceous | 86.5 ± 7 a | 91.0 ± 16 ab | 99.2 ± 1.2 b | 97.5 ± 4 ab | 99.6 ± 0.8 b | 96.9 ± 11 ab | 87 ± 11 ab |
| T. densiflora R | 80.3 ± 6 a | 87.7 ± 13 ab | 92.9 ± 2 bc | 96.3 ± 2 c | 98.1 ± 3 c | 98.8 ± 1 c | 92.5 ± 3 bc |
| T. densiflora A | 79.0 ± 9 a | 85.9 ± 9 a | 93.2 ± 3 ab | 98.9 ± 2 b | 98.1 ± 2 b | 95.6 ± 3.2 b | 88.0 ± 3 ab |
| Extract | % Immobility J2s | |||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | |
| A. aurantium A | 44.1 ± 6 a | 54.02 ± 7 b | 68.8 ± 5 b | 68.3± 6 b | 73.8 ± 6.1 ab | 70.8 ± 10 b |
| A. aurantium R | 40.1 ± 14 a | 34.1 ± 6 ab | 52.1 ± 6 b | 63.1 ± 4 c | 70.2 ± 6 c | 71.3 ± 7 c |
| A. cuspidata | 44.3 ± 15 b | 29.3 ± 7 a | 19.6 ± 9 a | 25.2 ± 10 a | 64.2 ± 5 c | 73.7 ± 4 c |
| A. integrifolium | 17.6 ± 8 a | 42.0 ± 14 b | 62.31 ± 11 c | 75.3 ± 4 c | 68.0 ± 10 c | 72.7 ± 10 c |
| A. subviscida | 31.4 ± 13 ab | 46.0 ± 10 abc | 29.6 ± 19 ab | 24.50 ± 4 a | 44.4 ± 26 bc | 60.2 ± 17 c |
| G. mexicanum | 20.1 ± 8 a | 28.8 ± 5 a | 26.4 ± 8 a | 40.4 ± 6 b | 58.4 ± 11 c | 66.9 ± 9 c |
| H. terebinthinaceous | 31.1 ± 10 a | 62.9 ± 8 bc | 62.9 ± 8 bc | 61.0 ± 5 b | 69.4 ± 6 bc | 73.6 ± 3 c |
| T. densiflora R | 46.5 ± 20 bc | 23.2 ± 12 a | 33.0 ± 8 ab | 49.1 ± 6 cd | 63.2 ± 8 de | 66.7 ± 10 e |
| T. densiflora A | 45.9 ± 9 a | 35.7 ± 16 a | 36.5 ± 15 a | 37.0 ± 11 a | 52.7 ± 17 ab | 64.6 ± 13 b |
| Extract | EC50,24h µg mL−1 | EC50,48h µg mL−1 |
|---|---|---|
| A. aurantium A | 53.5–187.4 | 31.5–110.4 |
| A. aurantium R | 289.4–468.1 | 63.2–88.3 |
| A. cuspidata | 132.1–282.8 | 54.1–199.3 |
| A. integrifolium | 71.4–214.1 | 47.4–107.1 |
| A. subviscida | 124.7–342.2 | 60.0–299.8 |
| G. mexicanum | 93.0–319.6 | 74.4–183.4 |
| H. terebinthinaceous | 84.5–183.4 | 56.0–164.4 |
| T. densiflora R | 170.3–301.8 | 59.0–112.3 |
| T. densiflora A | 91.3–184.7 | 81.9–166.1 |
| Fluopyram | 27.8–28.4 | 25.0–26.4 |
| Abamectin | 30.6–31.3 | 26.9–27.4 |
| Treatment | Concentration µg mL−1 | % Immobility | % Immobility after Washing |
|---|---|---|---|
| β-sitosterol | 100 † | 68.7 ± 8.5 a | 68.12 ± 8.0 |
| stigmasterol/β-sitosterol | 100 | 88.3 ± 8.1 b | --- |
| α-terthienyl | 100 | 93.3 ± 3.1 bc | -- |
| 100 † | 90.6 ± 5.60 bc | 89.72 ± 5.5 | |
| stigmasterol | 100 | 100.0 ± 0.0 c | -- |
| 100 † | 94.5 ± 5.3 bc | 93.85 ± 5.6 |
| Atom | 9 | 10 | 11 | |||
|---|---|---|---|---|---|---|
| δ 1H | δ 13C | δ 1H | δ 13C | δ 1H | δ 13C | |
| 1 | 10.54 s | - | 133.5 | - | 132.9 | |
| 2 | - | 157.8 | 5.8 d (1.3 Hz) | 122.9 | 5.80 bs | 121.4 |
| 3 | 8.05 s | - | 4.15 d (14.7 Hz) | 60.4 | 4.28 d (16.0 Hz) | 77.8 |
| 3.77 d (14.7 Hz) | 4.56 so | |||||
| 4 | - | 174.1 | - | - | - | - |
| 5 | 5.24 d (8.0) | 62.9 | 3.61 so | 53.4 | 3.71 so | 68.5 |
| 3.09 ddd (6.4 Hz) | 3.60 so | |||||
| 6 | 6.89 d (8.0) | - | 1.98 so | 35.8 | 2.45 bs | 34.8 |
| 1.98 so | 1.92 so | |||||
| 7 | - | 157.2 | 4.43 bs | 68.9 | 4.57 so | 69.8 |
| 8 | 5.79 s | - | 4.61 bs | 77.8 | 4.57 so | 95.5 |
| 9 | - | - | 4.78 bs 4.78 bs | 60.3 | 4.78 d (14 Hz) 4.72 d (14 Hz) | 60.9 |
| 10 | - | - | - | 166.9 | - | 167.1 |
| 11 | - | - | - | 127.2 | - | 127.6 |
| 12 | - | - | 6.18 qq (1.3, 7.3) | 138.3 | 6.16 qq (1.6, 8.4 Hz) | 139.0 |
| 13 | - | - | 1.87 q (1.3) | 20.5 | 1.87 q (1.6 Hz) | 20.1 |
| 14 | - | - | 1.93 dq (1.3, 7.3) | 15.9 | 1.95 dq (1.6, 8.4 Hz) | 16.0 |
| Specie (Family) | Collection Site | Voucher Number | Part Plant Used | Extraction Solvent |
|---|---|---|---|---|
| Acalypha cuspidata Jacq. (Euphorbiaceae) | B | 25068 | Stem | MeOH |
| Acalypha subviscida S. Watson var. Lovelanddii McVaugh (Euphorbiaceae) | A | 24007 | Stem | MeOH |
| Alloispermum integrifolium (DC.) H. Rob. (Asteraceae) | A | 24024 | Stem | MeOH |
| Adenophyllum aurantium (L.) Strother (Asteraceae) | C | 25173 | Stem Root | MeOH MeOH |
| Galium mexicanum Kunth (Rubiaceae) | A | 23994 | Stem | MeOH |
| Heliocarpus terebinthinaceus (DC.) Hochr. (Tiliaceae) | D | 25225 | Seeds | H2O |
| Tournefortia densiflora M. Martens & Galeotti (Boraginaceae) | C | 25221 | Stem Root | MeOH MeOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Azorsa, R.; Cruz-Santiago, H.; Cid del Prado-Vera, I.; Ramirez-Mares, M.V.; Gutiérrez-Ortiz, M.d.R.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Lira-de León, K.I.; Hernández-Carlos, B. Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential. Molecules 2021, 26, 2216. https://doi.org/10.3390/molecules26082216
Velasco-Azorsa R, Cruz-Santiago H, Cid del Prado-Vera I, Ramirez-Mares MV, Gutiérrez-Ortiz MdR, Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Lira-de León KI, Hernández-Carlos B. Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential. Molecules. 2021; 26(8):2216. https://doi.org/10.3390/molecules26082216
Chicago/Turabian StyleVelasco-Azorsa, Raúl, Héctor Cruz-Santiago, Ignacio Cid del Prado-Vera, Marco Vinicio Ramirez-Mares, María del Rocío Gutiérrez-Ortiz, Norma Francenia Santos-Sánchez, Raúl Salas-Coronado, Claudia Villanueva-Cañongo, Karla Isabel Lira-de León, and Beatriz Hernández-Carlos. 2021. "Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential" Molecules 26, no. 8: 2216. https://doi.org/10.3390/molecules26082216
APA StyleVelasco-Azorsa, R., Cruz-Santiago, H., Cid del Prado-Vera, I., Ramirez-Mares, M. V., Gutiérrez-Ortiz, M. d. R., Santos-Sánchez, N. F., Salas-Coronado, R., Villanueva-Cañongo, C., Lira-de León, K. I., & Hernández-Carlos, B. (2021). Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential. Molecules, 26(8), 2216. https://doi.org/10.3390/molecules26082216