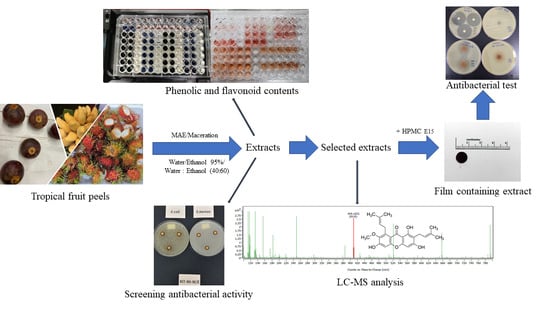
Extraction of Tropical Fruit Peels and Development of HPMC Film Containing the Extracts as an Active Antibacterial Packaging Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tropical Fruit Peels Extraction
2.2. Phenolic Content of the Fruit Peel Extracts
2.3. Xanthones and Flavonoid Content of the Fruit Peel Extracts
2.4. Antibacterial Property of the Fruit Peel Extracts
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Selected Extracts
2.6. Characteristics of the Films
2.7. Mechanical Property of the Films
2.8. Antibacterial Property of Films Containing Extracts
3. Materials and Methods
3.1. Materials
3.2. Tropical Fruits Extraction
3.2.1. Tropical Fruit Peel Collection
3.2.2. Microwave-Assisted Extraction
3.2.3. Maceration
3.3. Phenolic Content Analysis
3.4. Flavonoid Content Analysis
3.5. Antibacterial Activity Test of Extracts
3.6. Compounds Identification
3.6.1. Sample Preparation
3.6.2. LC-MS Analysis
3.7. Preparation of the Film Containing Extract
3.8. Characterization of the Films
3.9. Mechanical Property Test
3.10. Antibacterial Activity of Films Containing Extracts
3.11. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food. Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Vichitrananda, S.; Somsri, S. Tropical fruit production in thailand. Acta Hortic. 2008, 787, 33–46. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Zhou, D.; Rakariyatham, N.; Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods 2020, 67, 103846. [Google Scholar] [CrossRef]
- Janardhanan, S.; Mahendra, J.; Girija, A.S.S.; Mahendra, L.; Priyadharsini, V. Antimicrobial Effects of Garcinia Mangostana on Cariogenic Microorganisms. J. Clin. Diagn. Res. 2017, 11, ZC19–ZC22. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G.A. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for integral exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction Methods of Citrus Peel Phenolic Compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Mauriello, G. Chapter 11—Control of Microbial Activity Using Antimicrobial Packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 141–152. [Google Scholar]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control. 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [Green Version]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Abang Zaidel, D.N.; Muhamad, I.I.; Abdul Hamid, M.; Yaakob, H.; Mohd Jusoh, Y.M. Optimization of the antioxidant-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon 2019, 5, e02571. [Google Scholar] [CrossRef] [Green Version]
- Chaovanalikit, A.; Mingmuang, A.; Kitbunluewit, T.; Choldumrongkool, N.; Sondee, J.; Chupratum, S. Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of Mangosteen products. Int. Food Res. J. 2012, 19, 1047–1053. [Google Scholar]
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Kuan, A.-W.; Guo, H.-R.; Lin, Y.-H.; Wang, P.-K. Free Radical-Scavenging, Anti-Inflammatory, and Antibacterial Activities of Water and Ethanol Extracts Prepared from Compressional-Puffing Pretreated Mango (Mangifera indica L.) Peels. J. Food Qual. 2018, 2018, 1025387. [Google Scholar] [CrossRef] [Green Version]
- Castro-Vargas, H.I.; Ballesteros Vivas, D.; Ortega Barbosa, J.; Morantes Medina, S.J.; Aristizabal Gutiérrez, F.; Parada-Alfonso, F. Bioactive Phenolic Compounds from the Agroindustrial Waste of Colombian Mango Cultivars ’Sugar Mango’ and ’Tommy Atkins’-An Alternative for Their Use and Valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeing, J.S.; Barizão, E.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monrroy, M.; Araúz, O.; García, J.R. Active Compound Identification in Extracts of N. lappaceum Peel and Evaluation of Antioxidant Capacity. J. Chem. 2020, 2020, 4301891. [Google Scholar] [CrossRef] [Green Version]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of Mango (Mangifera indica L.) Cultivars for Their Contents of Flavonol O- and Xanthone C-Glycosides, Anthocyanins, and Pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef]
- Suttirak, W.; Manurakchinakorn, S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014, 51, 3546–3558. [Google Scholar] [CrossRef] [Green Version]
- Thitilertdecha, N.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules 2010, 15, 1453–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yang, Z.; Tang, B.; Zhang, Q.; Chen, Z.; Zhang, J.; Wei, J.; Sun, L.; Yan, J. Identification of Xanthones from the Mangosteen Pericarp that Inhibit the Growth of Ralstonia solanacearum. ACS Omega 2020, 5, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Klangmuang, P.; Sothornvit, R. Barrier properties, mechanical properties and antimicrobial activity of hydroxypropyl methylcellulose-based nanocomposite films incorporated with Thai essential oils. Food Hydrocoll. 2016, 61, 609–616. [Google Scholar] [CrossRef]
- Kvitek, O.; Mutylo, E.; Vokata, B.; Ulbrich, P.; Fajstavr, D.; Reznickova, A.; Svorcik, V. Photochemical Preparation of Silver Colloids in Hydroxypropyl Methylcellulose for Antibacterial Materials with Controlled Release of Silver. Coatings 2020, 10, 1046. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Khosrowshahi Asl, A. Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose—A review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondaveeti, S.; Damato, T.C.; Carmona-Ribeiro, A.M.; Sierakowski, M.R.; Petri, D.F.S. Sustainable hydroxypropyl methylcellulose/xyloglucan/gentamicin films with antimicrobial properties. Carbohyd. Polym. 2017, 165, 285–293. [Google Scholar] [CrossRef]
- Smieja, M. Current indications for the use of clindamycin: A critical review. Can. J. Infect. Dis. 1998, 9, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Kim, C.T.; Kim, C.J.; Cho, Y.J.; Chun, B.Y.; Lee, S.J.; Cha, J.Y.; Kim, T.H. Preparation of starch and cellulose-basededible films incorporated with propolis extract and theirphysical and antimicrobial properties. Food Eng. Prog. 2005, 9, 1–7. [Google Scholar]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-Mangostin-Rich Extracts from Mangosteen Pericarp: Optimization of Green Extraction Protocol and Evaluation of Biological Activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, H.; Zhuang, Y. Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. J. Food Sci. 2012, 77, C198–C204. [Google Scholar] [CrossRef]
- Gusman, J.; Tsai, P.-J. Extraction of Antioxidant Compounds from Rambutan (Nephelium lappaceum L.) Peel as Agricultural Waste in Taiwan. J. Trop. Crop Sci. 2015, 2, 10–16. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007, 100, 451–458. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de FFSoares, N.; Mattoso, L.H. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef] [PubMed]






| Fruit Peel | Extraction Method | Solvent | Yield (%) |
|---|---|---|---|
| Mangosteen | Water | 17.02 ± 2.40 a | |
| Maceration | Ethanol 95% | 16.98 ± 1.90 a | |
| Water–ethanol (40:60) | 24.98 ± 1.78 bc | ||
| MAE | Water | 17.32 ± 0.17 a | |
| Ethanol 95% | 16.42 ± 3.08 a | ||
| Water–ethanol (40:60) | 22.84 ± 1.24 bc | ||
| Mango | Water | 48.60 ± 0.28 d | |
| Maceration | Ethanol 95% | 25.84 ± 1.81 bc | |
| Water–ethanol (40:60) | 46.08 ± 0.34 e | ||
| MAE | Water | 45.32 ± 4.41 de | |
| Ethanol 95% | 26.68 ± 0.17 b | ||
| Water–ethanol (40:60) | 44.92 ± 0.87 de | ||
| Rambutan | Water | 26.68 ± 1.07 b | |
| Maceration | Ethanol 95% | 26.92 ± 1.41 b | |
| Water–ethanol (40:60) | 34.92 ± 0.96 f | ||
| Water | 28.05 ± 2.60 b | ||
| MAE | Ethanol 95% | 27.36 ± 2.26 b | |
| Water–ethanol (40:60) | 34.58 ± 2.97 f |
| Extract | Growth Inhibition Zone (mm) | |||
|---|---|---|---|---|
| 100 mg/mL | 10 mg/mL | |||
| S. aureus | E. coli | S. aureus | E. coli | |
| MT-MAE-W | NA | NA | NA | NA |
| MT-MAE-E | 9.19 ± 0.34 c1 | 8.94 ± 0.39 bcf# | 8.50 ± 0.11 b1 | 9.18 ± 0.05 b# |
| MT-MAE-W/E | 9.46 ± 0.20 aj1 | 9.88 ± 0.21 ag# | 8.07 ± 0.08 a2 | 8.89 ± 0.16 a* |
| MT-Ma-W | NA | NA | NA | NA |
| MT-Ma-E | 9.54 ± 0.25 acej1 | 9.24 ± 0.17 bd# | 8.36 ± 0.06 ab2 | 8.00 ± 0.14 d* |
| MT-Ma-W/E | 9.65 ± 0.07 acd1 | 9.22 ± 0.41 abf# | 8.24 ± 0.47 ab1 | 8.29 ± 0.06 c* |
| MG-MAE-W | NA | NA | NA | NA |
| MG-MAE-E | 9.41 ± 0.25 acfj1 | 7.67 ± 0.38 e | 8.33 ± 0.35 a2 | NA |
| MG-MAE-W/E | 9.63 ± 0.19 acj1 | 8.83 ± 0.50 bf | 8.69 ± 0.25 b2 | NA |
| MG-Ma-W | NA | NA | NA | NA |
| MG-Ma-E | 9.83 ± 0.46 agj1 | 9.35 ± 0.65 bfg | 9.67 ± 0.46 c1 | NA |
| MG-Ma-W/E | 9.94 ± 0.32 bdegj1 | 8.55 ± 0.29 cdf | 9.17 ± 0.06 c2 | NA |
| RT-MAE-W | 7.95 ± 0.32 h | NA | NA | NA |
| RT-MAE-E | 10.68 ± 0.16 bi | NA | NA | NA |
| RT-MAE-W/E | 10.29 ± 0.25 bg | NA | NA | NA |
| RT-Ma-W | 11.01 ± 0.25 i | NA | NA | NA |
| RT-Ma-E | 10.10 ± 0.66 bdg | NA | NA | NA |
| RT-Ma-W/E | 10.07 ± 0.33 bdfgj | NA | NA | NA |
| Clindamycin HCl solution (1%) | 37.228 ± 0.564 k1 | 32.092 ± 0.483 h# | 37.043 ± 0.606 d1 | 32.666 ± 0.419 e# |
| 100% DMSO | NA | NA | NA | NA |
| Film Sample | Thickness (mm ± SD) | Weight (g ± SD) | Diameter (mm) |
|---|---|---|---|
| MT-MAE-W/E film | 0.852 ± 0.028 a | 0.050 ± 0.003 a | 7.94 |
| MT-Ma-W/E film | 0.848 ± 0.037 a | 0.047 ± 0.005 a | 7.94 |
| Blank film | 0.335 ± 0.024 b | 0.021 ± 0.001 b | 7.94 |
| Film | Tensile Strength (N/mm2) | Elongation at Break (%) | Young’s Modulus (N/mm2) |
|---|---|---|---|
| MT-MAE-W/E film | 2.71 ± 0.30 a | 0.68 ± 0.10 a | 20.48 ± 1.57 a |
| MT-Ma-W/E film | 2.64 ± 0.42 a | 0.60 ± 0.06 a | 20.44 ± 1.28 a |
| Blank film | 4.79 ± 1.57 b | 8.72 ± 2.09 b | 25.40 ± 2.75 b |
| Samples | Growth Inhibition Zone (mm) | |
|---|---|---|
| S. aureus | E. coli | |
| Blank film | NZ | NZ |
| MT- MAE-W/E film | 30.22 ± 2.14 a1 | 26.50 ± 1.60 a1 |
| MT- Ma-W/E film | 30.60 ± 2.83 a1 | 26.93 ± 3.92 a1 |
| Clindamycin HCl solution | 34.76 ± 0.10 b1 | 16.58 ± 0.60 b2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Kantrong, N.; Sommano, S.R.; Rachtanapun, P.; Junmahasathien, T.; Kumpugdee-Vollrath, M.; Jantrawut, P. Extraction of Tropical Fruit Peels and Development of HPMC Film Containing the Extracts as an Active Antibacterial Packaging Material. Molecules 2021, 26, 2265. https://doi.org/10.3390/molecules26082265
Chaiwarit T, Kantrong N, Sommano SR, Rachtanapun P, Junmahasathien T, Kumpugdee-Vollrath M, Jantrawut P. Extraction of Tropical Fruit Peels and Development of HPMC Film Containing the Extracts as an Active Antibacterial Packaging Material. Molecules. 2021; 26(8):2265. https://doi.org/10.3390/molecules26082265
Chicago/Turabian StyleChaiwarit, Tanpong, Nutthapong Kantrong, Sarana Rose Sommano, Pornchai Rachtanapun, Taepin Junmahasathien, Mont Kumpugdee-Vollrath, and Pensak Jantrawut. 2021. "Extraction of Tropical Fruit Peels and Development of HPMC Film Containing the Extracts as an Active Antibacterial Packaging Material" Molecules 26, no. 8: 2265. https://doi.org/10.3390/molecules26082265
APA StyleChaiwarit, T., Kantrong, N., Sommano, S. R., Rachtanapun, P., Junmahasathien, T., Kumpugdee-Vollrath, M., & Jantrawut, P. (2021). Extraction of Tropical Fruit Peels and Development of HPMC Film Containing the Extracts as an Active Antibacterial Packaging Material. Molecules, 26(8), 2265. https://doi.org/10.3390/molecules26082265