Crystal Engineering of Schiff Base Zn(II) and Cd(II) Homo- and Zn(II)M(II) (M = Mn or Cd) Heterometallic Coordination Polymers and Their Ability to Accommodate Solvent Guest Molecules
Abstract
:1. Introduction
2. Results
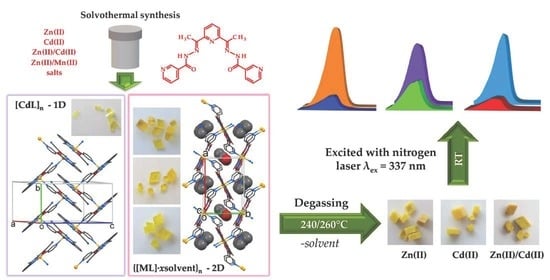
2.1. Synthesis Aspects, Crystal Structure and Thermal Characterization
2.2. Photoluminescent Properties of CPs 1–5
2.3. Investigation of Crystal Properties after Guest Molecules Degassing
3. Materials and Methods
3.1. Synthesis of Coordination Polymers
3.2. Crystallographic Data Collection and Refinements
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design. Analysis and Application; RSC: Cambridge, UK, 2009. [Google Scholar]
- Ortiz, O.L.; Ramirez, L.D. Coordination Polymers and Metal Organic Frameworks; Nova Science Publishers: New York, NY, USA, 2012; Chapter 7; pp. 225–247. [Google Scholar]
- García, H.; Navalón, S. (Eds.) Metal-Organic Frameworks: Applications in Separations and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- MacGillivray, L.R.; Lukehart, C.M. Metal-Organic Framework Materials; MacGillivray, L.R., Lukehart, C.M., Eds.; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804. [Google Scholar] [CrossRef]
- Heine, J.; Muller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Mironov, V.S.; Yakushev, I.A.; Svetogorov, R.D.; Maximova, O.V.; Manakin, Y.V.; Kornev, A.B.; Vasiliev, A.N.; Yagubskii, E.B. End-to-end azido-bridged lanthanide chain complexes (Dy, Er, Gd, and Y) with a pentadentate Schiff-base [N3O2] ligand: Synthesis, structure, and magnetism. Inorg. Chem. 2020, 59, 563–578. [Google Scholar] [CrossRef]
- Pichon, C.; Elrez, B.; Béreau, V.; Duhayon, C.; Sutter, J.-P. From heptacoordinated CrIII complexes with cyanide or isothiocyanate apical groups to 1D heterometallic assemblages with all-pentagonal-bipyramid coordination geometries. Eur. J. Inorg. Chem. 2018, 2018, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Naskar, S.; Corbella, M.; Blake, A.J.; Chattopadhyay, S.K. Versatility of 2,6-diacetylpyridine (dap) hydrazones in generating varied molecular architectures: Synthesis and structural characterization of a binuclear double helical Zn(II) complex and a Mn(II) coordination polymer. Dalton Trans. 2007, 11, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.K.; Gogoi, N.; Pichon, C.; Durga Prasad Goli, V.M.L.; Thlijeni, M.; Duhayon, C.; Suaud, N.; Guihery, N.; Barra, A.-L.; Ramasesha, S.; et al. Pentagonal bipyramid FeII complexes: Robust ising-spin units towards heteropolynuclear nanomagnets. Chem. Eur. J. 2017, 23, 4380–4396. [Google Scholar] [CrossRef]
- Bretosh, K.; Béreau, V.; Duhayon, C.; Pichon, C.; Sutter, J.-P. A ferromagnetic Ni(II)–Cr(III) single-chain magnet based on pentagonal bipyramidal building units. Inorg. Chem. Front. 2020, 7, 1503–1511. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Pichon, C.; Suaud, N.; Duhayon, C.; Guihéry, N.; Sutter, J.-P. Cyano-bridged Fe(II)–Cr(III) single-chain magnet based on pentagonal bipyramid units: On the added value of aligned axial anisotropy. J. Am. Chem. Soc. 2018, 140, 7698–7704. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Gogoi, N.; Duhayon, C.; Ramasesha, S.; Sutter, J.-P. Single-ion magnet behaviour of heptacoordinated Fe(II) complexes: On the importance of supramolecular organization. Chem. Commun. 2015, 51, 3616–3619. [Google Scholar] [CrossRef]
- Dey, M.; Sarma, B.; Gogoi, N. Coligand promoted controlled assembly of hierarchical heterobimetallic nitroprusside based aggregates. Z. Anorg. Allg. Chem. 2014, 640, 2962–2967. [Google Scholar] [CrossRef]
- Kopotkov, V.A.; Sasnovskaya, V.D.; Korchagin, D.V.; Morgunov, R.B.; Aldoshin, S.M.; Simonov, S.V.; Zorina, L.V.; Schaniel, D.; Woike, T.; Yagubskii, E.B. The first photochromic bimetallic assemblies based on Mn(III) and Mn(II) Schiff-base (salpn, dapsc) complexes and pentacyanonitrosylferrate. CrystEngComm 2015, 17, 3866–3876. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Sasnovskaya, V.D.; Talantsev, A.D.; Morgunov, R.B.; Mironov, V.S.; Yagubskii, E.B. Slow magnetic relaxation, antiferromagnetic ordering, and metamagnetism in MnII(H2dapsc)-FeIII(CN)6 chain complex with highly anisotropic Fe-CN-Mn spin coupling. Chem. Eur. J. 2019, 25, 14583–14597. [Google Scholar] [CrossRef] [PubMed]
- Sasnovskaya, V.D.; Kopotkov, V.A.; Talantsev, A.D.; Morgunov, R.B.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Mironov, V.S. Synthesis, structure, and magnetic properties of 1D {[MnIII(CN)6][MnII(dapsc)]}n coordination polymers: Origin of unconventional single-chain magnet behaviour. Inorg. Chem. 2017, 56, 8926–8943. [Google Scholar] [CrossRef]
- Naskar, S.; Mishra, D.; Chattopadhyay, S.K.; Corbella, M.; Blake, A.J. Versatility of 2,6-diacetylpyridine (dap) hydrazones in stabilizing uncommon coordination geometries of Mn(II): Synthesis, spectroscopic, magnetic and structural characterization. Dalton Trans. 2005, 21, 2428–2435. [Google Scholar] [CrossRef]
- Li, Z.-W.; Wang, X.; Wei, L.-Q.; Ivanović-Burmazović, I.; Liu, G.-F. Subcomponent self-assembly of covalent metallacycles templated by catalytically active seven-coordinate transition metal centers. J. Am. Chem. Soc. 2020, 142, 7283–7288. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Miao, H.; Mei, H.; Zhou, S.; Xu, Y. Two novel bi-functional hybrid materials constructed from POMs and a Schiff base with excellent third-order NLO and catalytic properties. Dalton Trans. 2016, 45, 7947–7951. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-X.; Li, H.-Q.; Miao, H.; Shao, D.; Wei, X.-Q.; Shi, L.; Zhang, Y.-Q.; Wang, X.-Y. Heterometallic MIILnIII (M=Co/Zn; Ln=Dy/Y) complexes with pentagonal bipyramidal 3d centers: Syntheses, structures, and magnetic properties. Inorg. Chem. 2018, 57, 15526–15536. [Google Scholar] [CrossRef]
- Konar, S.; Jana, A.; Das, K.; Ray, S.; Chatterjee, S.; Golen, J.A.; Rheingold, A.L.; Kar, S.K. Synthesis, crystal structure, spectroscopic and photoluminescence studies of manganese(II), cobalt(II), cadmium(II), zinc(II) and copper(II) complexes with a pyrazole derived Schiff base ligand. Polyhedron 2011, 30, 2801–2808. [Google Scholar] [CrossRef]
- Rodriguez-Arguelles, M.C.; Ferrari, M.B.; Fava, G.G.; Pelizzi, C.; Tarasconi, P.; Albertini, R.; Dall’Aglio, P.P.; Lunghi, P.; Pinelli, S. 2,6-Diacetylpyridine bis(thiosemicarbazones) zinc complexes: Synthesis, structure, and biological activity. J. Inorg. Biochem. 1995, 58, 157–175. [Google Scholar] [CrossRef]
- Danilescu, O.; Bulhac, I.; Shova, S.; Novitskii, G.; Bourosh, P. Coordination compounds of copper(II) with Schiff bases based on aromatic carbonyl compounds and hydrazides of carboxylic acids: Synthesis, structures, and properties. Russ. J. Coord. Chem. 2020, 46, 838–849. [Google Scholar] [CrossRef]
- Bulhac, I.; Danilescu, O.; Rija, A.; Shova, S.; Kravtsov, V.C.; Bourosh, P. Cobalt(II) complexes with pentadentate Schiff bases 2,6-diacetylpyridine hydrazones: Syntheses and structures. Russ. J. Coord. Chem. 2017, 43, 21–36. [Google Scholar] [CrossRef]
- Bourosh, P.; Bulhac, I.; Mirzac, A.; Shova, S.; Danilescu, O. Mono- and dinuclear Vanadium complexes with the pentadentate Schiff base 2,6-diacetylpyridine bis(nicotinylhydrazone): Synthesis and structures. Russ. J. Coord. Chem. 2016, 42, 157–165. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; John Wiley & Sons: Hoboken, NJ, USA, 1957. [Google Scholar]
- Gudasi, K.B.; Patil, S.A.; Vadavi, R.S.; Shenoy, R.V.; Nethaji, M.; Bligh, S.W.A. Synthesis and spectral investigation of manganese(II), cadmium(II) and oxovanadium(IV) complexes with 2,6-diacetylpyridine bis(2-aminobenzoylhidrazone): Crystal structure of manganese(II), and cadmium(II) complexes. Inorg. Chim. Acta 2006, 359, 3229–3236. [Google Scholar] [CrossRef]
- Fondo, M.; Sousa, A.; Bermejo, M.R.; Garcia-Deibe, A.; Sousa-Pedrares, A.; Hoyos, O.L.; Helliwell, M. Electrochemical synthesis and X-ray characterisation of cadmium complexes containing 2,6-bis(1-salicyloylhidrazonoethyl)pyridine—The influence of the supporting electrolyte on the nature of the isolated compounds. Eur. J. Inorg. Chem. 2002, 2002, 703–710. [Google Scholar] [CrossRef]
- Pelizzi, C.; Pelizzi, G.; Vitali, F. Investigation into aroylhydrazones as chelating agents. Part 8. Synthesis and spectroscopic characterization of complexes of Co, Ni, Cu, Zn, and Cd with 2,6-diacetylpyridine bis(salicyloylhydrazone); X-ray crystal structure of dichloro[2,6-diacetylpyridine bis(salicyloylhydrazone)]cadmium(II)-chloroform-methanol (1/1/1). J. Chem. Soc. Dalton Trans. 1987, 1, 177–181. [Google Scholar]
- Nithya, P.; Simpson, J.; Govindarajan, S. Syntheses, structural diversity and thermal behavior of first row transition metal complexes containing potential multidentate ligands based on 2,6-diacetylpyridine and benzyl carbazate. Polyhedron 2018, 141, 5–16. [Google Scholar] [CrossRef]
- Tyula, Y.A.; Zabardasti, A.; Goudarziafshar, H.; Roudsari, M.S.; Dusek, M.; Eigner, V. Template synthesis of two new supramolecular zinc(II) complexes containing pentadentate N3O2 semicarbazone ligand: Nanostructure synthesis, Hirshfeld surface analysis, and DFT studies. J. Mol. Struct. 2017, 1150, 383–394. [Google Scholar] [CrossRef]
- Van de Walle, A.; Tiwary, P.; de Jong, M.; Olmsted, D.L.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Andjelkovic, K.; Sumar, M.; Ivanovic-Burmazovic, I. Thermal analysis in structural characterization of hydrazone ligands and their complexes. J. Therm. Anal. Calorim. 2001, 66, 759–778. [Google Scholar] [CrossRef]
- Qin, Y.; She, P.; Huang, X.; Huang, W.; Zhao, Q. Luminescent manganese(II) complexes: Synthesis, properties and optoelectronic applications. Coord. Chem. Rev. 2020, 416, 213331. [Google Scholar] [CrossRef]
- Drzewiecki, A.; Padlyak, B.; Adamiv, V.; Burak, Y.; Teslyuk, I. EPR spectroscopy of Cu2+ and Mn2+ in borate glasses. Nukleonika 2013, 58, 379–385. [Google Scholar]
- Qin, B.-W.; Zhang, X.-Y.; Zhang, J.-P. A stable multifunctional cadmium-organic framework based on 2D stacked layers: Effective gas adsorption, and excellent detection of Cr3+, CrO42−, and Cr2O72−. Dyes Pigment. 2020, 174, 108011. [Google Scholar] [CrossRef]
- Hua, J.-A.; Zhao, Y.; Kang, Y.-S.; Lu, Y.; Sun, W.-Y. Solvent-dependent zinc(II) coordination polymers with mixed ligands: Selective sorption and fluorescence sensing. Dalton Trans. 2015, 44, 11524–11532. [Google Scholar] [CrossRef] [PubMed]
- Chisca, D.; Croitor, L.; Petuhov, O.; Kulikova, O.V.; Volodina, G.F.; Coropceanu, E.B.; Masunov, A.E.; Fonari, M.S. Tuning structures and emissive properties in a series of Zn(II) and Cd(II) coordination polymers containing dicarboxylic acids and nicotinamide pillars. CrystEngComm 2018, 20, 432–447. [Google Scholar] [CrossRef]
- Kravtsov, V.C.; Lozovan, V.; Siminel, N.; Coropceanu, E.B.; Kulikova, O.V.; Costriucova, N.V.; Fonari, M.S. From 1D to 2D Cd(II) and Zn(II) coordination networks by replacing monocarboxylate with dicarboxylates in partnership with azine ligands: Synthesis, crystal structures, inclusion, and emission properties. Molecules 2020, 25, 5616. [Google Scholar] [CrossRef]
- Mazza, P.; Zani, F.; Orcesi, M.; Pelizzi, C.; Pelizzi, G.; Predieri, G. Synthesis, structure, antimicrobial, and genotoxic activities of organotin compounds with 2,6-diacetylpyridine nicotinoyl- and isonicotinoylhydrazones. J. Inorg. Biochem. 1992, 48, 251–270. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.J.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]









| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | C21H17Cd1N7O2 | C45H45Cd2N11O11 | C45H51Zn2N15O8 | C45H42Cd1.25Zn0.75N15O5.5 | C45H47MnZnN15O8 |
| Formula weight | 511.82 | 566.38 | 530.37 | 1070.46 | 1046.28 |
| Crystal system | Monoclinic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | C2/c | P21212 | P21212 | P21212 | P21212 |
| a (Å) | 28.6789(18) | 13.6165(4) | 12.4717(3) | 9.4973(4) | 9.4825(6) |
| b (Å) | 8.9005(6) | 9.4836(4) | 9.4383(3) | 13.3860(6) | 12.6536(8) |
| c (Å) | 16.0789(11) | 9.7867(3) | 10.1540(3) | 9.8617(4) | 10.0787(8) |
| β (°) | 104.375(7) | 90 | 90 | 90 | 90 |
| V (Å3) | 3975.8(5) | 1263.79(8) | 1195.24(6) | 1253.71(9) | 1209.32(14) |
| Z | 8 | 2 | 2 | 1 | 1 |
| ρcalc (g cm−3) | 1.710 | 1.488 | 1.474 | 1.418 | 1. 437 |
| μMo (mm−1) | 1.134 | 0.904 | 1.073 | 0.948 | 0.825 |
| F(000) | 2048 | 572 | 550 | 544 | 541 |
| Crystal size (mm) | 0.42 × 0.14 × 0.08 | 0.40 × 0.30 × 0.20 | 0.30 × 0.25 × 0.25 | 0.22 × 0.22 × 0.22 | 0.38 × 0.38 × 0.22 |
| Reflections collected/unique | 6602/3698 (Rint = 0.0587) | 3172/2241 (Rint = 0.0225) | 2963/1841 (Rint = 0.0237) | 3151/2040 (Rint = 0.0303) | 3062/1812 (Rint = 0.0332) |
| Completeness (%) | 99.8 | 99.6 | 99.2 | 98.9 | 99.2 |
| Reflections with I > 2σ(I) | 2471 | 2059 | 1686 | 1745 | 1596 |
| Parameters | 282 | 161 | 166 | 161 | 161 |
| GOF | 1.000 | 1.007 | 1.000 | 1.007 | 1.008 |
| R1, wR2 (I > 2σ(I)) | 0.0532, 0.0997 | 0.0367, 0.0995 | 0.0367, 0.0960 | 0.0453, 0.1175 | 0.0472, 0.1215 |
| R1, wR2 (all data) | 0.0876, 0.1156 | 0.0414, 0.1038 | 0.0417, 0.0994 | 0.0562, 0.1268 | 0.0563, 0.1287 |
| Δρmax/Δρmin (e⋅Å−3) | 0.771/−0.807 | 0.976/−0.463 | 0.476/−0.454 | 0.775/−0.305 | 0.601/−0.314 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilescu, O.; Bourosh, P.N.; Petuhov, O.; Kulikova, O.V.; Bulhac, I.; Chumakov, Y.M.; Croitor, L. Crystal Engineering of Schiff Base Zn(II) and Cd(II) Homo- and Zn(II)M(II) (M = Mn or Cd) Heterometallic Coordination Polymers and Their Ability to Accommodate Solvent Guest Molecules. Molecules 2021, 26, 2317. https://doi.org/10.3390/molecules26082317
Danilescu O, Bourosh PN, Petuhov O, Kulikova OV, Bulhac I, Chumakov YM, Croitor L. Crystal Engineering of Schiff Base Zn(II) and Cd(II) Homo- and Zn(II)M(II) (M = Mn or Cd) Heterometallic Coordination Polymers and Their Ability to Accommodate Solvent Guest Molecules. Molecules. 2021; 26(8):2317. https://doi.org/10.3390/molecules26082317
Chicago/Turabian StyleDanilescu, Olga, Paulina N. Bourosh, Oleg Petuhov, Olga V. Kulikova, Ion Bulhac, Yurii M. Chumakov, and Lilia Croitor. 2021. "Crystal Engineering of Schiff Base Zn(II) and Cd(II) Homo- and Zn(II)M(II) (M = Mn or Cd) Heterometallic Coordination Polymers and Their Ability to Accommodate Solvent Guest Molecules" Molecules 26, no. 8: 2317. https://doi.org/10.3390/molecules26082317
APA StyleDanilescu, O., Bourosh, P. N., Petuhov, O., Kulikova, O. V., Bulhac, I., Chumakov, Y. M., & Croitor, L. (2021). Crystal Engineering of Schiff Base Zn(II) and Cd(II) Homo- and Zn(II)M(II) (M = Mn or Cd) Heterometallic Coordination Polymers and Their Ability to Accommodate Solvent Guest Molecules. Molecules, 26(8), 2317. https://doi.org/10.3390/molecules26082317