Effect of Salt Stress on Growth and Metabolite Profiles of Cape Gooseberry (Physalis peruviana L.) along Three Growth Stages
Abstract
:1. Introduction
2. Results
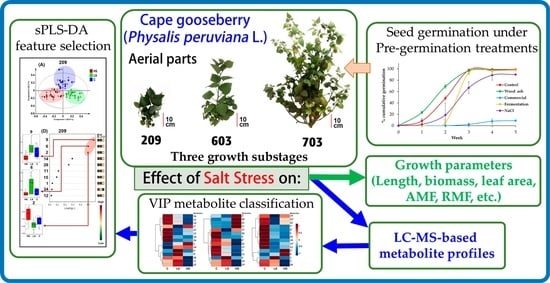
2.1. Selection of Pre-Germination Treatment
2.2. Effects of Salt Stress on Growth of P. peruviana
2.3. Effects of Salt Stress on Metabolic Profiles of P. peruviana Aerial Parts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Germination Assays:
4.3. Plant Material and Management
4.4. Plant Treatments
4.5. Growth Parameters
4.6. Extraction of Plant Material
4.7. LC-MS Analysis, Annotation, and Identification of Top-Ranked Metabolites
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Nutrient | mg/L |
|---|---|
| Ca(NO3)2 | 861.14 |
| KNO3 | 418.85 |
| Agrofeed (MgNO3) | 0.142 * |
| MgSO4 | 382.88 |
| NH4H2PO4 | 119.23 |
| Quelafeed Fe | 0.03 * |
| Mf Mn ** | 4.17 |
| Mf Cu ** | 0.33 |
| Kelatex Zn ** | 0.78 |
| Quibor ** | 1.67 |
| (NH4)6Mo7O24 | 0.089 |
| # | Rt (min) | [M − H]− (m/z) | Annotation a | # | Rt (min) | [M − H]− (m/z) | Annotation a |
|---|---|---|---|---|---|---|---|
| 1 | 2.8 | 245 | hispidin | 15 | 27.0 | 533 | withangulatin isomer |
| 2 | 3.4 | 301 | quercetin b | 16 | 27.4 | 503 | dihydroixocarpalactone isomer |
| 3 | 12.8 | 367 | feruloylquinic acid | 17 | 30.5 | 721 | physagulin isomer 1 |
| 4 | 13.4 | 609 | quercetin rhamnosyl-glucoside | 18 | 31.6 | 559 | unidentified withanolide 1 |
| 5 | 17.6 | 755 | kaempferol rhamnosyl-diglucoside | 19 | 32.3 | 555 | unidentified withanolide 2 |
| 6 | 18.6 | 771 | quercetin 3-O-β-robinobioside-7-O-β-glucoside b | 20 | 33.0 | 411 | alkesterol isomer |
| 7 | 20.1 | 485 | withaphysanolide b | 21 | 33.3 | 815 | unknown |
| 8 | 20.7 | 499 | physanolide A b | 22 | 34.4 | 515 | unidentified withanolide 3 |
| 9 | 21.3 | 593 | quercetin 3-O-β-glucosyl(l→6)-β-galactoside b | 23 | 35.3 | 619 | physagulin D b |
| 10 | 23.2 | 547 | physalin isomer | 24 | 37.1 | 981 | glycosylated triterpene |
| 11 | 23.4 | 625 | quercetin diglucoside | 25 | 37.6 | 705 | physagulin isomer 2 |
| 12 | 24.1 | 521 | deoxyphysalolactone isomer | 26 | 39.2 | 809 | unknown |
| 13 | 24.4 | 473 | pubescenol | 27 | 39.8 | 735 | unidentified withanolide 4 |
| 14 | 26.8 | 509 | physalin B b | 28 | 41.4 | 719 | unidentified withanolide 5 |
References
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M., Hasegawa, P., Jain, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–32. [Google Scholar]
- Singh, B.; Mishra, S.; Bohra, A.; Joshi, R.; Siddique, K. Crop phenomics for abiotic stress tolerance in crop plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 277–296. [Google Scholar]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant 2016, 38, 72. [Google Scholar] [CrossRef]
- Miranda, D.; Fischer, G.; Mewis, I.; Rohn, S.; Ulrichs, C. Salinity effects on proline accumulation and total antioxidant activity in leaves of the cape gooseberry (Physalis peruviana L.). J. Appl. Bot. Food Qual. 2014, 87, 67–73. [Google Scholar]
- Yu, L.; Fan, J.; Xu, C. Peroxisomal fatty acid β-oxidation negatively impacts plant survival under salt stress. Plant Signal. Behav. 2019, 14, 1561121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc. Publishers: Los Angeles, CA, USA, 2010; Volume 1. [Google Scholar]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Hollman, P.C.H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Zheng, J.; Yu, Z.-C.; Huang, X.-D.; Zhang, Q.-L.; Tian, X.-S.; Peng, C.-L. Functional characteristics of phenolic compounds accumulated in young leaves of two subtropical forest tree species of different successional stages. Tree Physiol. 2018, 38, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Mascarenhas De Souza, C.; Oliveira De Souza, M.; Muniz de Oliveira, L.; Pelacani, C.; Regina Pelacani, C. Effect of priming on germinability and salt tolerance in seeds and seedlings of Physalis peruviana L. Afr. J. Biotechnol. 2014, 13, 1955–1960. [Google Scholar]
- Maruenda, H.; Cabrera, R.; Cañari-Chumpitaz, C.; Lopez, J.; Toubiana, D. NMR-based metabolic study of fruits of Physalis peruviana L. grown in eight different Peruvian ecosystems. Food Chem. 2018, 262, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): An overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- Fernandes, F.; Rodrigues, S.; Law, C.; Mujumdar, A. Drying of exotic tropical fruits: A comprehensive review. Food Bioprocess. Technol. 2011, 4, 163–185. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.; Dekker, M.; Verkerk, R.; van Boekel, M. Health-promoting compounds in cape gooseberry (Physalis peruviana L.): Review from a supply chain perspective. Trends Food Sci. Technol. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Jones, P.; Varady, K. Are functional foods redefining nutritional requirements? Appl. Physiol. Nutr. Metab. 2008, 33, 118–123. [Google Scholar] [CrossRef]
- Puente, L.; Pinto-Muñoz, C.; Castro, E.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Muniz, J.; Kretzschmar, A.; Rufato, L.; Pelizza, T.; Rufato, A.; de Macedo, T. General aspects of physalis cultivation. Cienc. Rural 2014, 44, 964–970. [Google Scholar] [CrossRef]
- Fischer, G.; Almanza-Merchán, P.; Miranda, D. Importancia y cultivo de la Uchuva (Physalis peruviana L.). Rev. Bras. Frutic. 2014, 36, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Perea, M.; Rodríguez, N.; Fischer, G.; Velásquez, M.; Micán, Y. Cape gooseberry Physalis peruviana L. (Solanaceae) [text in Spanish]. In Biotechnology Applied to the Improvement of Tropical Fruit Crops; Universidad Nacional de Colombia: Bogotá, Colombia, 2010; pp. 466–490. [Google Scholar]
- Çakir, Ö.; Pekmez, M.; Çepnİ, E.; Candar, B.; Fİdan, K. Evaluation of biological activities of Physalis peruviana ethanol extracts and expression of Bcl-2 genes in HeLa cells. Food Sci. Technol. 2014, 34, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Kasali, F.; Kadima, J.; Mpiana, P.; Ngbolua, K.; Tshibangu, D. Assessment of antidiabetic activity and acute toxicity of leaf extracts from Physalis peruviana L. in guinea-pig. Asian Pac. J. Trop. Biomed. 2013, 3, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Arun, M.; Asha, V. Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J. Ethnopharmacol. 2007, 111, 110–114. [Google Scholar] [CrossRef]
- Mazorra, M.; Quintana, A.; Miranda, D.; Fischer, G.; Valencia, M. Aspectos anatómicos de la formación y crecimiento del fruto de uchuva. Acta Biol. Colomb. 2006, 11, 69–81. [Google Scholar]
- Herrera, M.; Fischer, G.; Chacón, M. Agronomical evaluation of cape gooseberries (Physalis peruviana L.) from central and north-eastern Colombia. Agron. Colomb. 2012, 30, 15–24. [Google Scholar]
- Fischer, G.; Melgarejo, L. The ecophysiology of cape gooseberry (Physalis peruviana L.)—an Andean fruit crop. A review. Rev. Colomb. Cienc. Hortic. 2020, 14, 76–89. [Google Scholar] [CrossRef]
- Miranda, D.; Fischer, G.; Ulrichs, C. Growth of cape gooseberry (Physalis peruviana L.) plants affected by salinity. J. Appl. Bot. Food Qual. 2010, 83, 175–181. [Google Scholar]
- Llano, S.; Muñoz-Jiménez, A.; Jiménez-Cartagena, C.; Londoño-Londoño, J.; Medina, S. Untargeted metabolomics reveals specific withanolides and fatty acyl glycoside as tentative metabolites to differentiate organic and conventional Physalis peruviana fruits. Food Chem. 2018, 244, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, X.; Zheng, Q. Metabolomic profiling of carotenoid constituents in Physalis peruviana during different growth stages by LC-MS/MS technology. J. Food Sci. 2019, 00, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Nakamura, M.; Suzuki, H.; Yamazaki, M.; Knoch, E.; Mori, T.; Umemoto, N.; Morita, M.; Hirai, G.; Sodeoka, M.; et al. Comparative characterization of the leaf tissue of physalis alkekengi and Physalis peruviana using RNA-seq and metabolite profiling. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, F.; Fischer, G.; Davenport, T.; Pinzón, J.; Ulrichs, C. Cape gooseberry (Physalis peruviana L.) phenology according to the BBCH phenological scale. Sci. Hortic. 2013, 162, 39–42. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [Green Version]
- Filippou, P.; Bouchagier, P.; Skotti, E.; Fotopoulos, V. Proline and reactive oxygen/nitrogen species metabolism is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ. Exp. Bot. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khanam, S.; Hasan, S.; Ali, B.; Hayat, S.; Ahmad, A. Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol. Plant 2009, 31, 889–897. [Google Scholar] [CrossRef]
- Maas, E.; Poss, J. Salt sensitivity of wheat at various growth stages. Irrig. Sci. 1989, 10, 29–40. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Dursun, A. Salt tolerance of Physalis during germination and seedling growth. Pak. J. Bot. 2011, 43, 2673–2676. [Google Scholar]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Dhanvantari, B. Effect of seed extraction methods and seed treatments on control of tomato bacterial canker. Can. J. Plant Pathol. 1989, 11, 400–408. [Google Scholar] [CrossRef]
- Mabundza, R.; Wahome, P.; Masarirambi, M. Effects of different pre-germination treatment methods on the germination of passion (Passiflora edulis) seeds. J. Agric. Soc. Sci. 2010, 6, 57–60. [Google Scholar]
- Dickens, D. Effect of fruit fermentation on the germination and growth of Irvingia wombolu (Vermoesen) seedlings. Am. J. Biotechnol. Mol. Sci. 2011, 1, 45–50. [Google Scholar]
- Kochanek, J.; Long, R.; Lisle, A.; Flematti, G. Karrikins identified in biochars indicate post-fire chemical cues can influence community diversity and plant development. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celikli, F.; Akkelle, P.; Onus, A. In vitro salinity evaluation studies in golden berry (Physalis peruviana L.). Biotechnol. J. Int. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Kent, L.; Läuchli, A. Germination and seedling growth of cotton: Salinity–calcium interactions. Plant Cell Environ. 1985, 8, 155–159. [Google Scholar] [CrossRef]
- Esringü, A.; Kant, C.; Yildirim, E.; Karlidag, H.; Turan, M. Ameliorative effect of foliar nutrient supply on growth, inorganic ions, membrane permeability, and leaf relativewater content of physalis plants under salinity stress. Commun. Soil Sci. Plant Anal. 2011, 42, 408–423. [Google Scholar] [CrossRef]
- Nasri, N.; Saïdi, I.; Kaddour, R.; Lachâal, M. Effect of salinity on Arabidopsis thaliana seed germination and acid phosphatase activity. Arch. Biol. Sci. 2016, 68, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Miranda, D.; Ulrichs, C.; Fischer, G. Dry matter accumulation and foliar K, Ca and Na contents in salt-stressed cape gooseberry (Physalis peruviana L.) plants. Agron. Colomb. 2010, 28, 165–172. [Google Scholar]
- Fischer, G.; Almanza-Merchán, P.; Ramírez, F. Source-sink relationships in fruit species: A review. Rev. Colomb. Cienc. Hortícolas 2013, 6, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, Y.; Zhang, S.; Zhang, J. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 2002, 53, 2201–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Zhu, J. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Flórez, F.; López-Cristoffanini, C.; Jáuregui, O.; Melgarejo, L.M.; López-Carbonell, M. Changes in ABA, IAA and JA levels during calyx, fruit and leaves development in cape gooseberry plants (Physalis peruviana L.). Plant Physiol. Biochem. 2017, 115, 174–182. [Google Scholar] [CrossRef]
- Ahmed, I.; Nadira, U.; Bibi, N.; Cao, F.; He, X.; Zhang, G.; Wu, F. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ. Exp. Bot. 2015, 111, 1–12. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Isaac, C.; Jiménez, E.; Yane, E.; Martínez, C.; Fonseca, J. Flavonoids and their antioxidant actions [text in spanish]. Rev. Fac. Med. 2009, 52, 73–75. [Google Scholar]
- Li, P.; Li, Y.; Zhang, F.; Zhang, G.; Jiang, X.; Yu, H.; Hou, B. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Şahin, G. Effects of salicylic acid and heat acclimation on thermotolerance and withanolide accumulation under high temperature stress in the Cape gooseberry (Physalis peruviana L.). Turk. J. Bot. 2019, 43, 468–474. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, A.M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanveer, M.; Shabala, S. Targeting redox regulatory mechanisms for salinity stress tolerance in crops. In Salinity Responses and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 213–234. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Orozco, L.; Trillos, O.; Cotes, J. Evaluation of two methodologies in cape gooseberry (Physalis peruviana L.) seed extraction [text in Spanish]. Rev. Fac. Cienc. Básicas 2010, 6, 52–65. [Google Scholar]
- Miranda, D.; Ulrichs, C.; Fischer, G. Imbibition and percentage of germination of cape gooseberry (Physalis peruviana L.) seeds under NaCl stress. Agron. Colomb. 2010, 28, 29–35. [Google Scholar]
- Villar, R.; Ruiz-Robleto, J.; Quero, J.; Poorter, H.; Valladares, F.; Marañón, T. Growth rates in woody species: Functional aspects and ecological implications [text in spanish]. In Mediterranean Forest Ecology in a Changing World; EGRAF S.A.: Madrid, Spain, 2004; pp. 191–227. [Google Scholar]
- Benedetto, A.; Tognetti, J. Plant growth analysis techniques: Their application to intensive crops [text in Spanish]. Rev. Investig. Agropecu. 2016, 42, 258–282. [Google Scholar]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Batista-Navarro, R.T. Natural Product Resources BT—Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 1499–1501. ISBN 978-1-4419-9863-7. [Google Scholar]
- Nawwar, M.A.M.; El-Mousallamy, A.M.D.; Barakat, H.H. Quercetin 3-glycosides from the leaves of Solanum nigrum. Phytochemistry 1989, 28, 1755–1757. [Google Scholar] [CrossRef]
- Brasseur, T.; Angenot, L. Six flavonol glycosides from leaves of Strychnos variabilis. Phytochemistry 1988, 27, 1487–1490. [Google Scholar] [CrossRef]
- Ahmad, S.; Malik, A.; Yasmin, R.; Ullah, N.; Gul, W.; Khan, P.M.; Nawaz, H.R.; Afza, N. Withanolides from Physalis peruviana. Phytochemistry 1999, 50, 647–651. [Google Scholar] [CrossRef]
- Damu, A.G.; Kuo, P.-C.; Su, C.-R.; Kuo, T.-H.; Chen, T.-H.; Bastow, K.F.; Lee, K.-H.; Wu, T.-S. Isolation, structures, and structure−cytotoxic activity relationships of withanolides and physalins from Physalis angulata. J. Nat. Prod. 2007, 70, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Nagafuji, S.; Okawa, M.; Kinjo, J. Trypanocidal constituents in plants 6. 1) Minor withanolides from the aerial parts of Physalis angulata. Chem. Pharm. Bull. 2006, 54, 1226–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinforma. 2019, 68, e86. [Google Scholar] [CrossRef]



| Substage a | Condition b | Aerial Part | LA c (cm2) | Roots | TB c (g/g) | ||
|---|---|---|---|---|---|---|---|
| L c (cm) | B c (g) | L c (cm) | B c (g) | ||||
| 209 | C | 17.7 ± 1.0 E | 2.4 ± 0.7 C | 340 ± 98 C | 64.5 ± 3.5 BC | 1.2 ± 0.4 F | 3.7 ± 1.1 D |
| 209 | LS | 20.2 ± 1.0 E | 4.2 ± 1.2 C | 515 ± 149 C | 74.6 ± 2.2 A | 1.3 ± 0.4 F | 5.6 ± 1.6 D |
| 209 | HS | 20.5 ± 1.3 E | 2.4 ± 0.7 C | 371 ± 107 C | 70.0 ± 3.7 AB | 1.1 ± 0.3 F | 3.5 ± 1.0 D |
| 603 | C | 57.4 ± 1.5 C | 17.3 ± 5.0 BC | 1934 ± 558 B | 73.6 ± 3.3 A | 9.5 ± 2.8 C | 26.8 ± 7.7 CD |
| 603 | LS | 54.4 ± 2.6 C | 17.2 ± 5.0 BC | 1908 ± 551 B | 72.3 ± 2.5 A | 7.6 ± 2.2 CD | 24.8 ± 7.2 CD |
| 603 | HS | 45.2 ± 2.1 D | 10.9 ± 3.1 C | 1018 ± 352 C | 62.8 ± 2.8 BC | 4.6 ± 1.3 D | 15.5 ± 4.5 D |
| 703 | C | 92.3 ± 3.5 A | 53.2 ± 15.4 A | 3617 ± 1044 AB | 59.6 ± 1.8 C | 42.1 ± 9.1 A | 95.3 ± 27.5 A |
| 703 | LS | 81.6 ± 3.2 B | 45.0 ± 13.0 A | 3784 ± 1092 AB | 61.1 ± 1.3 C | 26.0 ± 5.5 B | 71.0 ± 20.5 AB |
| 703 | HS | 76.7 ± 3.4 B | 39.2 ± 11.3 AB | 4110 ± 1187 A | 60.0 ± 1.5 C | 15.2 ± 4.2 C | 54.3 ± 15.7 BC |
| Substage a | Condition b | R/A c (g/g) | LA/TB c (m2/kg) | AMF c (g/g) | RMF c (g/g) |
|---|---|---|---|---|---|
| 209 | C | 0.513 ± 0.145 | 9.3 ± 2.8 | 0.66 ± 0.20 | 0.34 ± 0.10 |
| 209 | LS | 0.317 ± 0.039 | 9.2 ± 1.7 | 0.76 ± 0.26 | 0.24 ± 0.04 |
| 209 | HS | 0.462 ± 0.126 | 10.6 ± 3.5 | 0.68 ± 0.24 | 0.32 ± 0.10 |
| 603 | C | 0.551 ± 0.174 | 7.2 ± 2.1 | 0.64 ± 0.19 | 0.36 ± 0.12 |
| 603 | LS | 0.438 ± 0.098 | 7.7 ± 2.4 | 0.70 ± 0.23 | 0.31 ± 0.08 |
| 603 | HS | 0.425 ± 0.142 | 7.9 ± 2.3 | 0.70 ± 0.21 | 0.30 ± 0.10 |
| 703 | C | 0.790 ± 0.449 | 3.8 ± 1.0 | 0.56 ± 0.18 | 0.44 ± 0.28 |
| 703 | LS | 0.579 ± 0.256 | 5.3 ± 1.4 | 0.63 ± 0.20 | 0.37 ± 0.18 |
| 703 | HS | 0.388 ± 0.178 | 7.6 ± 3.4 | 0.72 ± 0.22 | 0.28 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Velandia, D.; Coy-Barrera, E. Effect of Salt Stress on Growth and Metabolite Profiles of Cape Gooseberry (Physalis peruviana L.) along Three Growth Stages. Molecules 2021, 26, 2756. https://doi.org/10.3390/molecules26092756
Monroy-Velandia D, Coy-Barrera E. Effect of Salt Stress on Growth and Metabolite Profiles of Cape Gooseberry (Physalis peruviana L.) along Three Growth Stages. Molecules. 2021; 26(9):2756. https://doi.org/10.3390/molecules26092756
Chicago/Turabian StyleMonroy-Velandia, Daissy, and Ericsson Coy-Barrera. 2021. "Effect of Salt Stress on Growth and Metabolite Profiles of Cape Gooseberry (Physalis peruviana L.) along Three Growth Stages" Molecules 26, no. 9: 2756. https://doi.org/10.3390/molecules26092756
APA StyleMonroy-Velandia, D., & Coy-Barrera, E. (2021). Effect of Salt Stress on Growth and Metabolite Profiles of Cape Gooseberry (Physalis peruviana L.) along Three Growth Stages. Molecules, 26(9), 2756. https://doi.org/10.3390/molecules26092756