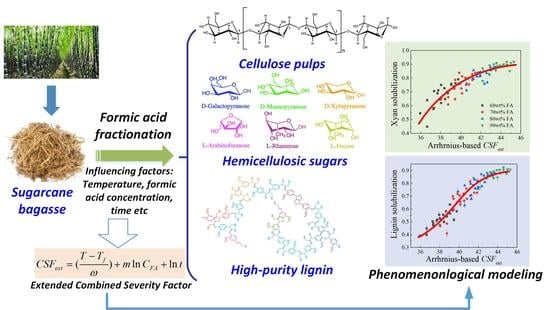
Phenomenological Modeling of Formic Acid Fractionation of Sugarcane Bagasse by Integration of Operation Parameters as an Extended Combined Severity Factor
Abstract
:1. Introduction
2. Results
2.1. Definition of Extended Combined Severity Factor and Phenomenological Modeling
2.2. Determination of Severity Constants and Model Parameters
2.3. Application of the Extended Severity Factor to Evaluate the Pretreatment Process
2.3.1. Use of CSFext as an Integrated Parameter to Correlate Operation Condition with Solubilization of Biomass Components
2.3.2. Evaluation of Enzymatic Digestibility of Cellulosic Solids
3. Discussion
4. Materials and Methods
4.1. Lignocellulosic Biomass and Chemicals
4.2. Formic Acid Fractionation of Sugarcane Bagasse
4.3. Enzymatic Hydrolysis of Pretreated Sugarcane Bagasse
4.4. Analytical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Morikawa, Y.; Zhao, X.; Liu, D. Biological co-production of ethanol and biodiesel from wheat straw: A case of dilute acid pretreatment. RSC Adv. 2014, 4, 37878–37888. [Google Scholar] [CrossRef]
- Ingle, A.P.; Chandel, A.K.; Antunes, F.A.F.; Mahendra, R.; da Silva, S.S. New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioprod. Biorefin. 2019, 13, 776–788. [Google Scholar] [CrossRef]
- Levya, P.F.; Sandersona, J.E.; Kisperta, R.G.; Wise, D.L. Biorefining of biomass to liquid fuels and organic chemicals. Enzyme Microb. Technol. 1981, 3, 207–215. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance.Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Li, M.; Yang, S.; Sun, R. Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour. Technol. 2016, 200, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Satyanagalakshmi, K.; Janu, K.U.; Sajna, K.V.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Formic Acid as a Potential Pretreatment Agent for the Conversion of Sugarcane Bagasse to Bioethanol. Appl. Biochem. Biotechnol. 2010, 162, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Dussana, K.; Girisuta, B.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. The effect of hydrogen peroxide concentration and solid loading on the fractionation of biomass in formic acid. Carbohydr. Polym. 2014, 111, 374–384. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.J.; Wild, P.J.; Gosselink, R.J.A.; Gerritsma, J.; Courtin, C.M. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Zhi, Z.; Qiao, Y.; Zhao, C.; Lu, X. Enhancement of Acidogenic Fermentation of Corn Stover Hydrolysates by Thermal Pretreatment with Diluted Formic Acid as Catalyst. Energy Fuels 2015, 29, 8157–8161. [Google Scholar] [CrossRef]
- Marzialetti, T.; Miller, S.J.; Jones, C.W.; Agrawal, P.K. Switchgrass pretreatment and hydrolysis using low concentrations of formic acid. J. Chem. Technol. Biot. 2011, 86, 706–713. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Chornet, E.; Belkacemi, K.; Overend, R.P. Phenomenological kinetics of complex systems-the development of a generalized severity parameter and its application to lignocellulosics fractionation. Chem. Eng. Sci. 1992, 47, 1109–1122. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatemnts. Philos. Trans. R Soc. Lond. A 1987, 321, 523–536. Available online: https://www.jstor.org/stable/37798 (accessed on 7 May 2021).
- Chum, H.L.; Johnson, D.K.; Black, S.K.; Overend, R.P. Pretreatment catalyst effects and the combined severity parameter. Appl. Biochem. Biotechnol. 1990, 24, 1–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Vancov, T.; Mackintosh, S.; Basu, B.; Lali, A.; Qian, G.; Hobson, P.; Doherty, W.O.S. Assessing dilute acid pretreatment of different lignocellulosic biomasses for enhanced sugar production. Cellulose 2016, 23, 3771–3783. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Sivagurunathan, P.; Kim, S.H. Effect of severity on dilute acid pretreatment of lignocellulosic biomass and the following hydrogen fermentation. Int. J. Hydrogen Energy 2016, 41, 21678–21684. [Google Scholar] [CrossRef]
- Iroba, K.L.; Tabil, L.G.; Sokhansanj, S.; Dumonceaux, T. Pretreatment and fractionation of barley straw using steam explosion at low severity factor. Biomass Bioenergy 2014, 66, 286–300. [Google Scholar] [CrossRef]
- Zoulikha, M.R.; Thierry, M.; Qiuyu, Z.J.M.; Nouviaire, A.; Ahmed, R.S. Combined steamexplosion toward vacuum and dilute-acid spraying of wheat straw. Impact of severity factor on enzymatic hydrolysis. Renew. Energy 2015, 78, 516–526. [Google Scholar] [CrossRef]
- Jang, S.; Kim, H.; Jeong, H.; Kim, J.; Yeo, H.; Choi, I.G. Effect of ethanol organosolv pretreatment factors on enzymatic digestibility and ethanol organosolv lignin structure from Liriodendron tulipifera in specifific combined severity factors. Renew. Energy 2016, 87, 599–606. [Google Scholar] [CrossRef]
- Goh, C.S.; Tan, H.T.; Lee, K.T.; Brosse, N. Evaluation and optimization of organosolv pretreatment using combined severity factors and response surface methodology. Biomass Bioenergy 2011, 35, 4025–4033. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Kinetic modeling and mechanisms of acid-catalyzed delignification of sugarcane bagasse by aqueous acetic acid. BioEnergy Res. 2013, 6, 436–447. [Google Scholar] [CrossRef]
- Dong, L.; Wu, R.; Zhao, X.; Liu, D. Phenomenological modeling and evaluation of formic acid pretreatment of wheat straw with an extended combined severity factor for biomass fractionation and enzymatic saccharification to produce bioethanol. J. Taiwan Inst. Chem. Eng. 2017, 81, 140–149. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, X.; Liu, D. Structural features of formiline pretreated sugar cane bagasse and their impact on the enzymatic hydrolysis of cellulose. Sustain. Chem. Eng. 2016, 4, 1255–1261. [Google Scholar] [CrossRef]
- Pan, X.; Gilkes, N.; Saddler, J. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Han, Y.; Bai, Y.; Zhang, J.; Liu, D.; Zhao, X. A comparison of different oxidative pretreatments on polysaccharide hydrolyzability and cell wall structure for interpreting the greatly improved enzymatic digestibility of sugarcane bagasse by delignification. BIOB 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Brännvall, E. The Limits of delignifification in Kraft cooking. BioResources 2017, 12, 2081–2107. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Liu, D. Relative significance of the negative impacts of hemicelluloses on enzymatic cellulose hydrolysis is dependent on lignin content: Evidence from substrate structural features and protein adsorption. ACS Sustain. Chem. Eng. 2016, 4, 6668–6679. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Multi-products co-production improves the economic feasibility of cellulosic ethanol: A case of Formiline pretreatment-based biorefining. Appl. Energy 2019, 250, 229–244. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–16. Available online: http://www.nrel.gov/biomass/analyticalprocedures.html#lap-002 (accessed on 7 May 2021).





| Model based on Arrhenius equation | Data Used for Fitting | Fitted Severity Parameters and Kinetic Constants | ||||||
| ω | m | a | b | R2 | F Value | p Value | ||
| Xylan solubilization | 22.53 | 8.23 | 0.1632 | −4.30 | 0.8337 | 127.02 | 0 | |
| Lignin solubilization | 11.06 | 16.10 | 0.1439 | −7.41 | 0.9076 | 295.74 | 0 | |
| Xyan plus lignin solubilizaiton | 0.1446 | 12.10 | 14.54 | −5.5595 | 0.9142 | 269.84 | 0 | |
| Total biomass solubilization | 0.1193 | 8.9665 | 78.4760 | −4.1200 | 0.5960 | 37.38 | 0 | |
| Model based on Logistic equation | Data Used for Fitting | ω | m | q | c | R2 | F Value | p Value |
| Xylan solubilization | 22.24 | 8.51 | 0.3093 | −7.88 | 0.8496 | 143.06 | 0 | |
| Lignin solubilization | 10.40 | 16.83 | 0.2459 | −12.60 | 0.9152 | 143.06 | 0 | |
| Xyan plus lignin solubilizaiton | 0.2640 | 12.52 | 13.91 | −9.9289 | 0.9210 | 295.26 | 0 | |
| Total biomass solubilization | 0.1536 | 9.1787 | 87.6569 | −4.9242 | 0.6003 | 38.05 | 0 | |
| No. | T (°C) | CFA (%) | t (h) | A-CSFext | L-CSFext | Experimental Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SY (%) | GC (%) | XC (%) | LC (%) | XR (%) | DD (%) | FC (%) | EGC @6h (%) | EGC @72h (%) | ||||||
| 1 | 80 | 60 | 0.25 | 35.95 | 37.11 | 68.7 | 56.17 ± 0.19 | 17.93 ± 0.09 | 21.16 ± 0.05 | 54.5 ± 0.3 | 39.06 ± 0.09 | 1.3 ± 0.03 | 4.1 ± 0.1 | 11.6 ± 0.1 |
| 2 | 80 | 70 | 0.5 | 38.75 | 39.97 | 64.7 | 63.7 ± 0.2 | 14.2 ± 0.2 | 18.9 ± 0.4 | 66.2 ± 0.5 | 48.6 ± 0.8 | 2.3 ± 0.01 | 6.0 ± 0.2 | 22.3 ± 2.5 |
| 3 | 80 | 80 | 1 | 41.27 | 42.56 | 51.6 | 74.2 ± 0.8 | 10.9 ± 0.06 | 13.6 ± 0.3 | 79.4 ± 0.1 | 70.7 ± 0.5 | 3.9 ± 0.02 | 4.8 ± 0.6 | 29.2 ± 0.4 |
| 4 | 80 | 90 | 1.5 | 43.29 | 44.63 | 43.4 | 77.9 ± 0.3 | 6.3 ± 0.05 | 6.7 ± 0.2 | 89.9 ± 0.1 | 88.3 ± 0.4 | 4.7 ± 0.04 | 3.2 ± 0.1 | 36.0 ± 0.2 |
| 5 | 90 | 60 | 0.5 | 37.33 | 38.52 | 64.7 | 52.4 ± 1.2 | 17.4 ± 0.3 | 19.4 ± 0.02 | 58.4 ± 0.8 | 47.5 ± 0.1 | 1.7 ± 0.02 | 10.4 ± 0.2 | 35.5 ± 3.3 |
| 6 | 90 | 70 | 0.25 | 38.74 | 40.00 | 79.1 | 60.4 ± 1.6 | 13.4 ± 0.02 | 13.9 ± 0.7 | 60.9 ± 0.1 | 54.6 ± 2.4 | 2.1 ± 0.05 | 10.7 ± 0.4 | 42.1 ± 3.8 |
| 7 | 90 | 80 | 1 | 41.96 | 43.28 | 49.6 | 64.6 ± 1.8 | 13.2 ± 0.4 | 10.7 ± 0.3 | 75.9 ± 0.6 | 77.9 ± 0.7 | 3.6 ± 0.05 | 8.0 ± 0.5 | 48.6 ± 2.2 |
| 8 | 90 | 90 | 1.5 | 43.97 | 45.35 | 45.9 | 71.6 ± 1.6 | 9.9 ± 0.01 | 5.9 ± 0.7 | 90.8 ± 0.01 | 89.2 ± 1.4 | 5.2 ± 0.1 | 3.9 ± 0.3 | 42.3 ± 2.9 |
| 9 | 99 | 60 | 1 | 38.64 | 39.86 | 63.0 | 69.4 ± 0.1 | 11.5 ± 0.06 | 17.3 ± 0.1 | 70.2 ± 0.1 | 58.3 ± 0.2 | 1.93 ± 0.01 | 8.6 ± 0.2 | 40.8 ± 1.1 |
| 10 | 99 | 70 | 1.5 | 41.15 | 42.45 | 46.0 | 74.5 ± 0.5 | 9.1 ± 0.09 | 10.8 ± 1.1 | 82.8 ± 0.2 | 81.5 ± 2.0 | 2.0 ± 0.02 | 6.1 ± 0.3 | 44.6 ± 0.2 |
| 11 | 99 | 80 | 0.25 | 41.19 | 42.54 | 46.1 | 73.7 ± 0.05 | 10.9 ± 0.2 | 11.8 ± 0.1 | 79.4 ± 0.4 | 79.8 ± 0.3 | 2.23 ± 0.001 | 6.2 ± 0.1 | 44.4 ± 2.1 |
| 12 | 99 | 90 | 0.5 | 32.28 | 44.90 | 54.2 | 81.5 ± 1.2 | 6.5 ± 0.1 | 7.3 ± 0.2 | 85.5 ± 0.2 | 85.5 ± 0.4 | 2.39 ± 0.2 | 4.9 ± 0.5 | 49.8 ± 1.4 |
| 13 | 105 | 60 | 1.5 | 39.46 | 40.70 | 63.0 | 65.9 ± 1.5 | 10.2 ± 0.2 | 18.0 ± 0.4 | 76.2 ± 0.5 | 51.6 ± 1.2 | 1.7 ± 0.04 | 12.2 ± 0.2 | 44.8 ± 0.6 |
| 14 | 105 | 70 | 1 | 41.16 | 42.46 | 53.6 | 69.7 ± 0.5 | 10.8 ± 0.02 | 13.1 ± 0.1 | 78.7 ± 0.04 | 70.5 ± 0.2 | 2.4 ± 0.02 | 7.9 ± 0.73 | 43.8 ± 6.8 |
| 15 | 105 | 80 | 0.5 | 42.30 | 43.66 | 46.7 | 83.0 ± 1.2 | 7.9 ± 0.1 | 7.6 ± 0.1 | 84.8 ± 0.2 | 87.1 ± 0.2 | 2.42 ± 0.3 | 5.9 ± 0.5 | 54.1 ± 0.5 |
| 16 | 105 | 90 | 0.25 | 43.21 | 44.64 | 46.7 | 81.4 ± 0.1 | 7.0 ± 0.03 | 6.7 ± 0.1 | 86.6 ± 0.1 | 88.7 ± 0.1 | 2.28 ± 0.001 | 5.4 ± 0.4 | 53.4 ± 1.0 |
| De-formylated Solid | Experimental Results | |||||||
|---|---|---|---|---|---|---|---|---|
| SY (%) | GC (%) | XC (%) | LC (%) | DD (%) | FC (%) | EGC@6h (%) | EGC@72h (%) | |
| Cellulosic solid obtained with 80% FA, 105 °C, 0.5 h | 55.0 | 80.2 ± 8.0 | 9.7 ± 0.9 | 9.3 ± 0.09 | 78.8 ± 0.2 | 0.22 ± 0.01 | 43.7 ± 1.6 | 72.1 ± 1.4 |
| Cellulosic solid obtained with 90% FA, 105 °C, 0.25 h | 53.1 | 82.7 ± 4.5 | 9.3 ± 0.5 | 8.0 ± 0.1 | 82.4 ± 0.3 | 0.39 ± 0.03 | 40.9 ± 1.8 | 74.9 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Zhang, J.; Wu, R.; Zhao, X. Phenomenological Modeling of Formic Acid Fractionation of Sugarcane Bagasse by Integration of Operation Parameters as an Extended Combined Severity Factor. Molecules 2021, 26, 2753. https://doi.org/10.3390/molecules26092753
Chang X, Zhang J, Wu R, Zhao X. Phenomenological Modeling of Formic Acid Fractionation of Sugarcane Bagasse by Integration of Operation Parameters as an Extended Combined Severity Factor. Molecules. 2021; 26(9):2753. https://doi.org/10.3390/molecules26092753
Chicago/Turabian StyleChang, Xiaogang, Jingzhi Zhang, Ruchun Wu, and Xuebing Zhao. 2021. "Phenomenological Modeling of Formic Acid Fractionation of Sugarcane Bagasse by Integration of Operation Parameters as an Extended Combined Severity Factor" Molecules 26, no. 9: 2753. https://doi.org/10.3390/molecules26092753
APA StyleChang, X., Zhang, J., Wu, R., & Zhao, X. (2021). Phenomenological Modeling of Formic Acid Fractionation of Sugarcane Bagasse by Integration of Operation Parameters as an Extended Combined Severity Factor. Molecules, 26(9), 2753. https://doi.org/10.3390/molecules26092753