In Vitro Antineoplastic and Antiviral Activity and In Vivo Toxicity of Geum urbanum L. Extracts
Abstract
:1. Introduction
2. Results
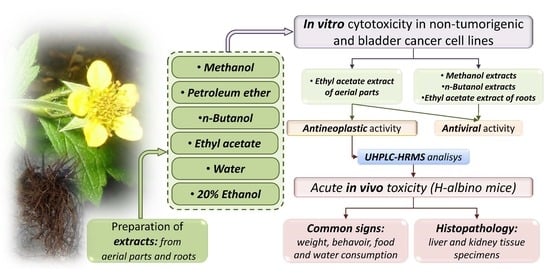
2.1. Experimental Design
2.2. In Vitro Cytotoxicity of G. urbanum Extracts on the Non-Tumorigenic Human Cell Line HEK293
2.3. Antiproliferative Effects of EtOAc-AP Extract from G. urbanum in Tumor Cell Lines
2.4. Pro-Apoptotic Effects of EtOAc-AP Extract of G. urbanum in Bladder Carcinoma Cell Lines
2.5. Antiviral Activity of Extracts from G. urbanum
2.6. UHPLC–HRMS Analysis of G. urbanum EtOAc-AP Extract
2.7. Acute In Vivo Toxicity of EtOAc-AP Extract
2.7.1. Common Signs of Acute Toxicity
2.7.2. Pathomorphological Evaluation of Liver Tissue Specimens
2.7.3. Pathomorphologial Evaluation of Kidney Tissue Specimens
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Preparation of G. urbanum Extracts
4.4. Cell Lines and Culture Conditions
4.5. Viruses
4.6. In Vitro Cytotoxicity Tests
4.6.1. MTT Test
4.6.2. Neutral Red Uptake Assay
4.7. Determination of Antiviral Activity
4.8. Colony-Forming Unit Assay
4.9. Total GSH Assay
4.10. Hoechst Staining
4.11. Caspase-3 Activity Assay
4.12. NFκB Assay
4.13. Ultra-High-Performace Liquid Chromatography—High-Resolution Mass Spectrometry (UHPLC–HRMS)
4.14. Acute Toxicity Test
4.15. Histopathology
4.16. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cheng, X.R.; Jin, H.Z.; Qin, J.J.; Fu, J.J.; Zhang, W.D. Chemical constituents of plants from the genus Geum. Chem. Biodivers. 2011, 8, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Granica, S.; Zwierzynska, M.; Stefanska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef]
- Yordanov, D.; Nikolov, P.; Boychinov, A. Phytotherapy (Book in Bulgarian); Medicine and Physical Education: Sofia, Bulgaria, 1970; p. 318. [Google Scholar]
- Tita, I.; Mogosanu, G.D.; Tita, M.G. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia 2009, 57, 141–156. [Google Scholar]
- Granica, S.; Klebowska, A.; Kosinski, M.; Piwowarski, J.P.; Dudek, M.K.; Kazmierski, S.; Kiss, A.K. Effects of Geum urbanum L. root extracts and its constituents on polymorphonuclear leucocytes functions. Significance in periodontal diseases. J. Ethnopharmacol. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine--an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [Green Version]
- Owczarek, A.; Gudej, J.; Kicel, A. Composition of essential oil from aerial and underground parts of Geum rivale and G. urbanum growing in Poland. Nat. Prod. Commun. 2013, 8, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton That, Q.; Nguyen Thien, T.V.; Dang, H.P.; Le Hoan, N.; Vo, L.K.T.; Nguyen, M.H.D.; Ngu, N.T.; Nguyen, T.S.; Hansen, P.E. Chemical constituents of Geum urbanum L. roots. Nat. Prod. Res. 2018, 32, 2529–2534. [Google Scholar] [CrossRef]
- Dimitrova, L.; Zaharieva, M.M.; Popova, M.; Kostadinova, N.; Tsvetkova, I.; Bankova, V.; Najdenski, H. Antimicrobial and antioxidant potential of different solvent extracts of the medicinal plant Geum urbanum L. Chem. Cent. J. 2017, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owczarek, A.; Gudej, J.; Olszewska, M. Antioxidant Activity of Geum rivale L. and Geum urbanum L. Acta Pol. Pharm. -Drug Res. 2015, 72, 1239–1244. [Google Scholar]
- Berkov, S.; Kasabova, N.; Pavlova, D.; Tonkov, S. Metabolic and chemotaxonomical studies in some Geum (Rosaceae) species. Phytol. Balc. 2017, 23, 7–16. [Google Scholar]
- Kurokawa, M.; Nagasaka, K.; Hirabayashi, T.; Uyama, S.-i.; Sato, H.; Kageyama, T.; Kadota, S.; Ohyama, H.; Hozumi, T.; Namba, T. Efficacy of traditional herbal medicines in combination with acyclovir against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 1995, 27, 19–37. [Google Scholar] [CrossRef]
- Panizzi, L.; Catalano, S.; Miarelli, C.; Cioni, P.L.; Campeol, E. In vitro antimicrobial activity of extracts and isolated constituents of Geum rivale. Phytother. Res. 2000, 14, 561–563. [Google Scholar] [CrossRef]
- Nikolova, M.; Valyovska-Popova, N.; Dimitrova, M.; Peev, D. High-mountain Bulgarian plants-free radical scavenging activity and flavonoid composition. J. BioSci. Biotechnol. 2014, SE, 29–33. [Google Scholar]
- Kaminska, J.; Assenow, I. Phytochemical studies of Geum bulgaricum Panc. Acta Pol. Pharm. Drug Res. 1971, 28, 201–206. [Google Scholar]
- Paun, G.; Neagu, E.; Albu, C.; Radu, G.L. Inhibitory potential of some Romanian medicinal plants against enzymes linked to neurodegenerative diseases and their antioxidant activity. Pharmacogn. Mag. 2015, 11, S110–S116. [Google Scholar] [CrossRef] [Green Version]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Yean, M.H.; Kim, J.S.; Hyun, Y.J.; Hyun, J.W.; Bae, K.H.; Kang, S.S. Terpenoids and phenolics from Geum japonicum. Korean J. Pharmacogn. 2012, 43, 107–121. [Google Scholar]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar] [PubMed]
- Dimitrova, L.; Philipov, S.; Zaharieva, M.M.; Miteva-Staleva, J.; Popova, M.; Tserovska, L.; Krumova, E.; Zhelezova, G.; Bankova, V.; Najdenski, H. In vivo assessment of acute and subacute toxicity of ethyl acetate extract from aerial parts of Geum urbanum L. Biotechnol. Biotechnol. Equip. 2021, 35, 61–73. [Google Scholar] [CrossRef]
- Dimitrova, L.; Popova, M.; Bankova, V.; Najdenski, H. Antiquorum Sensing Potential of Geum urbanum L. Comptes Rendus De L’acade’mie Bulg. Des Sci. 2019, 72, 341–349. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Franceschi, S. Infections and cancer: Established associations and new hypotheses. Crit Rev. Oncol. Hematol. 2009, 70, 183–194. [Google Scholar] [CrossRef]
- Moore, M.M.; Chua, W.; Charles, K.A.; Clarke, S.J. Inflammation and cancer: Causes and consequences. Clin. Pharm. 2010, 87, 504–508. [Google Scholar] [CrossRef]
- Sehdev, A.; Catenacci, D.V. Gastroesophageal cancer: Focus on epidemiology, classification, and staging. Discov. Med. 2013, 16, 103–111. [Google Scholar]
- Xie, F.J.; Zhang, Y.P.; Zheng, Q.Q.; Jin, H.C.; Wang, F.L.; Chen, M.; Shao, L.; Zou, D.H.; Yu, X.M.; Mao, W.M. Helicobacter pylori infection and esophageal cancer risk: An updated meta-analysis. World J. Gastroenterol. 2013, 19, 6098–6107. [Google Scholar] [CrossRef]
- Malnick, S.D.; Melzer, E.; Attali, M.; Duek, G.; Yahav, J. Helicobacter pylori: Friend or foe? World J. Gastroenterol. 2014, 20, 8979–8985. [Google Scholar] [CrossRef]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Bonefeld, C.M.; Wasik, M.A.; Koralov, S.B.; Geisler, C.; Kilian, M.; Iversen, L.; Woetmann, A.; et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins 2013, 5, 1402–1421. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, P.I.; Lorber, B.; Vonderheid, E.C. Infections complicating mycosis fungoides and Sezary syndrome. JAMA 1992, 267, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Almanza, G.; Ortiz-Sanchez, E.; Rocha-Zavaleta, L.; Rivas-Santiago, C.; Esparza-Ibarra, E.; Olmos, J. Cervical cancer stem cells and other leading factors associated with cervical cancer development. Oncol. Lett. 2019, 18, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, X. Seropositivity to herpes simplex virus type 2, but not type 1 is associated with cervical cancer: NHANES (1999–2014). BMC Cancer 2017, 17, 726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Park, H.K.; Park, S.; Lee, A.; Lee, Y.H.; Shin, D.Y.; Koh, Y.; Choi, J.Y.; Yoon, S.S.; Choi, Y.; et al. Strong association between herpes simplex virus-1 and chemotherapy-induced oral mucositis in patients with hematologic malignancies. Korean J. Intern. Med. 2019, 35, 1188. [Google Scholar] [CrossRef] [Green Version]
- Oeyen, E.; Hoekx, L.; De Wachter, S.; Baldewijns, M.; Ameye, F.; Mertens, I. Bladder Cancer Diagnosis and Follow-Up: The Current Status and Possible Role of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 821. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Global Cancer Facts & Figures, 3rd ed.; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Boivin, D.; Lamy, S.; Lord-Dufour, S.; Jackson, J.; Beaulieu, E.; Côté, M.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chem. 2009, 112, 374–380. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Swieca, M.; Dziki, D.; Seczyk, L.; Zlotek, U.; Rozylo, R.; Kaszuba, K.; Ryszawy, D.; Czyz, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. Biomed. Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef] [PubMed]
- Moscato, S.; Ronca, F.; Campani, D.; Danti, S. Poly(vinyl alcohol)/gelatin Hydrogels Cultured with HepG2 Cells as a 3D Model of Hepatocellular Carcinoma: A Morphological Study. J. Funct. Biomater. 2015, 6, 16–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Interagency Coordinating Committee on the Validation of Alternative Methods; National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity; National Institute of Environmental Health Sciences: North Carolina, NC, USA, 2001; Volume 01-4500. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ICS 11.100.20. International Organization for Standardization: London, UK, 2017.
- Donno, D.; Cavanna, M.; Beccaro, G.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.; Bounous, G. Currants and strawberries as bioactive compound sources: Determination of antioxidant profiles with HPLC-DAD/MS. J. Appl. Bot. Food Qual. 2013, 86, 1–10. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumara, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liang, C.; Li, C.; Bu, M.; Bu, L.; Xiao, Y.; Sun, H.; Zhang, L. Simultaneous Qualitative and Quantitative Study of Main Compounds in Commelina communis Linn. by UHPLC–Q-TOF-MS-MS and HPLC–ESI-MS-MS. J. Chromatogr. Sci. 2018, 56, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Sandjo, L.P.; Nascimento, M.V.; da Silva, L.A.; Munhoz, A.C.; Pollo, L.A.; Biavatti, M.W.; Ngadjui, B.T.; Opatz, T.; Fröde, T.S. ESI-MS(2) and Anti-inflammatory Studies of Cyclopropanic Triterpenes. UPLC-ESI-MS and MS(2) Search of Related Metabolites from Donella ubanguiensis. Phytochem. Anal. 2017, 28, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Cumberbatch, M.G.K.; Noon, A.P. Epidemiology, aetiology and screening of bladder cancer. Transl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef]
- Lemke, E.A.; Shah, A.Y. Management of Advanced Bladder Cancer: An Update. J. Adv. Pract. Oncol. 2018, 9, 410. [Google Scholar]
- Elsasser-Beile, U.; Leiber, C.; Wolf, P.; Lucht, M.; Mengs, U.; Wetterauer, U. Adjuvant intravesical treatment of superficial bladder cancer with a standardized mistletoe extract. J. Urol. 2005, 174, 76–79. [Google Scholar] [CrossRef]
- Urech, K.; Buessing, A.; Thalmann, G.; Schaefermeyer, H.; Heusser, P. Antiproliferative effects of mistletoe (Viscum album L.) extract in urinary bladder carcinoma cell lines. Anticancer Res. 2006, 26, 3049–3055. [Google Scholar]
- Lee, S.T.; Lu, M.H.; Chien, L.H.; Wu, T.F.; Huang, L.C.; Liao, G.I. Suppression of urinary bladder urothelial carcinoma cell by the ethanol extract of pomegranate fruit through cell cycle arrest and apoptosis. BMC Complement. Altern. Med. 2013, 13, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Paiva, P.; Gomes, F.; Napoleao, T.; Sa, R.; Correia, M.; Coelho, L.C. Antimicrobial Activity of Secondary Metabolites and Lectins from Plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2009, 1, 396–406. [Google Scholar]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Silacci, P.; Tretola, M. Pomegranate’s Ellagitannins: Metabolism and Mechanisms of Health Promoting Properties. Nutr. Food Sci Int J. 2019, 9. [Google Scholar] [CrossRef]
- Disis, M.L. Immune Regulation of Cancer. J. Clin. Oncol. 2010, 28, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer Immunosurveillance and Immunoediting: The Roles of Immunity in Suppressing Tumor Development and Shaping Tumor Immunogenicity. In Advances in Immunology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2006; Volume 90, pp. 1–50. [Google Scholar]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Katsman, A.; Umezawa, K.; Bonavida, B. Chemosensitization and immunosensitization of resistant cancer cells to apoptosis and inhibition of metastasis by the specific NF-kappaB inhibitor DHMEQ. Curr. Pharm. Des. 2009, 15, 792–808. [Google Scholar] [CrossRef] [PubMed]
- Oiso, S.; Ikeda, R.; Nakamura, K.; Takeda, Y.; Akiyama, S.; Kariyazono, H. Involvement of NF-kappaB activation in the cisplatin resistance of human epidermoid carcinoma KCP-4 cells. Oncol. Rep. 2012, 28, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, Y.; Chen, C.; Ma, J.; Sun, W.; Tian, Z.; Li, J.; Xu, J.; Liu, C.S.; Zhang, D.; et al. NF-κB p65 Overexpression Promotes Bladder Cancer Cell Migration via FBW7-Mediated Degradation of RhoGDIα Protein. Neoplasia 2017, 19, 672–683. [Google Scholar] [CrossRef]
- Lu, L.Y.; Ou, N.; Lu, Q.B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Rad. 2006, 5, 326–334. [Google Scholar]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Glutathione and apoptosis. Free Radic. Res. 2008, 42, 689–706. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, D.; Yakoub, A.M.; Yadavalli, T.; Agelidis, A.; Thakkar, N.; Hadigal, S.; Ames, J.; Shukla, D. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci. Transl. Med. 2018, 10, eaan5861. [Google Scholar] [CrossRef] [Green Version]
- Galvan D’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Sci. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J. Tissue Cult. Methods 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Kapoor, V.; Zaharieva, M.M.; Das, S.N.; Berger, M.R. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012, 319, 39–48. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Kirilov, M.; Chai, M.; Berger, S.M.; Konstantinov, S.; Berger, M.R. Reduced Expression of the Retinoblastoma Protein Shows That the Related Signaling Pathway Is Essential for Mediating the Antineoplastic Activity of Erufosine. PLoS ONE 2014, 9, e100950. [Google Scholar] [CrossRef]
- Konstantinov, S.M.; Eibl, H.; Berger, M.R. BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–380. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc. 2011, 1, pdb prot5557. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 4th ed.; Scion Publishing Ltd.: Banbury, UK, 2008. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Preparation of Cells and Tissues for Fluorescence Microscopy; Spector, D.L., Goldman, R.D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2006. [Google Scholar]






| Solvent | Part of the Plant | Parametersof the In Vitro Cytotoxicity Test | |||
|---|---|---|---|---|---|
| IC50 (µg/mL) | CI 95% * | m § | R # | ||
| Methanol | Aerial | 58.30 | 53.6–63.4 | −3.3 | 0.98 |
| Underground | 58.52 | 57.1–59.9 | −4.3 | 0.99 | |
| Ethyl acetate | Aerial | 62.30 | 60.7–63.9 | −7.3 | 0.99 |
| Underground | 97.35 | 93.4–101.5 | −5.3 | 0.99 | |
| n-Butanol | Aerial | 141.6 | 132.1–151.8 | −3.1 | 0.98 |
| Underground | 115.6 | 108.7–122.9 | −2.8 | 0.99 | |
| Petroleum ether | Aerial | 72.72 | 65.1–81.2 | −5.8 | 0.97 |
| Underground | 40.25 | 37.2–43.5 | −4.9 | 0.98 | |
| Water | Aerial | 379.4 | 343.6–419.0 | −7.9 | 0.98 |
| Underground | 296.0 | 265.5–330.0 | −3.8 | 0.96 | |
| 20% Ethanol | Aerial | 341.6 | 303.3–384.7 | −1.9 | 0.95 |
| Underground | 236.3 | 209.6–266.3 | −1.9 | 0.96 | |
| Solvent | Part of the Plant | Parameters of the In Vitro Cytotoxicity Test | ||||
|---|---|---|---|---|---|---|
| IC50 (µg/mL) | CI 95% * | m § | R # | SI ** | ||
| Methanol | Aerial | 79.20 | 68.1–92.1 | −1.5 | 0.95 | 0.74 |
| Underground | 84.30 | 72.7–97.7 | −0.9 | 0.96 | 0.69 | |
| Ethyl acetate | Aerial | 25.28 | 24.1–26.5 | −3.9 | 0.99 | 2.46 |
| Underground | 42.09 | 39.3–45.1 | −1.8 | 0.99 | 2.31 | |
| n-Butanol | Aerial | 201.3 | 178.2–227.4 | −1.2 | 0.97 | 0.70 |
| Underground | 170.4 | 155.5–186.8 | −1.5 | 0.98 | 0.68 | |
| Petroleum ether | Aerial | 70.15 | 60.6–81.2 | −1.6 | 0.95 | 0.83 |
| Underground | 41.49 | 38.8–44.3 | −2.4 | 0.99 | 0.97 | |
| Water | Aerial | 338.4 | 293.4–390.3 | −0.9 | 0.95 | 1.12 |
| Underground | 295.4 | 267.1–326.7 | −0.9 | 0.98 | 1.00 | |
| 20% Ethanol | Aerial | 323.5 | 253.9–412.1 | −0.8 | 0.92 | 1.06 |
| Underground | 343.4 | 281.0–419.6 | −1.3 | 0.90 | 0.69 | |
| Parameters of the In Vitro Cytotoxicity Test | Cell Lines | |
|---|---|---|
| BC-3C | HEP-G2 | |
| IC50 (µg/mL) | 21.33 | 76.81 |
| CI 95% * | 18.4–24.7 | 68.9–85.5 |
| m § | −0.8 | −1.4 |
| R # | 0.97 | 0.98 |
| SI ** | 2.92 | 0.81 |
| Extracts | CytotoxicityCC50 [µg/mL] | PV1 | CVB1 | CVB3 | HRSV-A2 | HAdV-5 | HSV 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEp 2 | MDBK | IC50 * | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | |
| n-BuOH-AP | 167 | 1050 | NA | - | NA | - | NA | - | NA | - | NA | - | 54 | 19.4 |
| n-BuOH-UP | 63 | 962 | NA | - | NA | - | NA | - | NA | - | NA | - | 28.3 | 34 |
| EtOAc-AP | 58.5 | 88.6 | NA | - | 57 | 1.07 | NA | - | NA | - | 10 | 5.8 | NA | - |
| EtOAc-UP | 39.7 | 320 | NA | - | NA | - | NA | - | NA | - | 2.2 | 18 | NA | - |
| MeOH-AP | 50 | 220 | NA | - | NA | - | NA | - | NA | - | NA | - | 24.7 | 8.9 |
| MeOH-UP | 83.5 | 1200 | NA | - | NA | - | NA | - | NA | - | 30 | 2.7 | 224.5 | 5.3 |
| NO. | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− | Fragmentation Pattern in (-) ESI-MS/MS | tR(min) | Δ ppm | Reference Standard (RS)/Reference |
|---|---|---|---|---|---|---|---|
| Gallic and Ellagic Acid Derivatives | |||||||
| 1. | gallic acid | C7H6O5 | 169.0132 | 169.0130 (34.67), 125.0228 (100), 97.0279 (3.25), 81.0331 (0.72), 69.0330 (4.70) | 1.18 | −7.257 | RS |
| 2. | ellagic acid | C14H6O8 | 300.9988 | 300.9991 (100), 245.0096 (1.81), 257.0087 (1.04), 229.0136 (3.67), 217.0139 (0.60), 201.0182 (3.83), 145.0282 (3.73), 117.0330 (1.04), | 9.03 | −0.500 | RS |
| 3. | gallic acid O-pentoside | C13H16O9 | 315.0728 | 315.0717 (7.32), 169.0131 (100), 125.0228 (39.01), 97.4442 (2.60) | 4.77 | 2.205 | Oszmiański et al., 2015 |
| 4. | galloylshikimic acid | C14H14O9 | 325.0565 | 325.0565 (46.17), 169.0131 (100), 137.0225 (4.63), 125.0228 (41.32), 111.0435 (8.16) | 1.73 | −0.047 | Singh et al., 2015 |
| 5. | galloylglucose | C13H16O10 | 331.0673 | 331.0673 (100), 271.0462 (14.20), 211.0241 (16.18), 169.0130 (71.64), 151.0021 (3.93), 125.0229 (38.49), 125.0229 (38.49), 113.0227 (2.62), 89.0229 (2.82), 71.0121 (4.67), 59.0122 (3.84) | 0.75 | −2.130 | Singh et al., 2015 |
| 6. | galloylglucose isomer | C13H16O10 | 331.0674 | 331.0674 (57.45), 271.0460 (87.40), 241.0351 (37.09), 211.0239 (30.67), 169.0130 (100), 161.0442 (5.55), 125.0228 (47.72), 107.0122 (7.29) | 1.13 | 1.027 | Singh et al., 2015 |
| 7. | ellagic acid O-pentoside | C14H6O8 | 433.0410 | 433.0413 (100), 300.9991 (67.43), 299.9912 (54.22), 245.0091 (0.82), 257.0091 (0.62), 229.0145 (2.03), 201.0199 (0.97) | 8.60 | −0.460 | Oszmiański et al., 2015 |
| 8. | ellagic acid O-deoxyhexoside | C20H16O12 | 447.0569 | 447.0577 (100), 300.9992 (66.28), 299.9915 (85.37) | 8.81 | −0.110 | Oszmiański et al., 2015 |
| 9. | ellagic acid O-hexoside | C20H16O13 | 463.0524 | 463.0525 (100), 300.9994 (67.48), 299.9904 (27.14) | 6.80 | 2.451 | |
| 10. | flavogallonic acid | C21H10O13 | 469.0029 | 469.0029 (10.53), 425.0157 (100), 299.9918 (92.60), 298.9836 (26.35), 135.2071 (4.21) | 6.21 | −4.123 | Singh et al., 2015 |
| 11. | ellagic acid O-hexuronide | C20H14O16 | 477.0312 | 477.0312 (57.99), 446.6355 (2.70), 300.9991 (100), 229.0140 (4.54), 244.5380 (3.12), 299.9882 (5.27) | 6.54 | 1.321 | |
| 12. | methylellagic acid O-hexoside | C21H18O13 | 477.0685 | 477.0685 (100), 315.0148 (72.30), 299.9916 (54.33), 298.9822 (8.02), 270.9877 (15.75) | 9.18 | 3.406 | |
| 13. | HHDP | C20H18O14 | 481.0628 | 481.0628 (100), 462.4490 (1.07), 300.9991 (77.43), 275.0194 (37.30), 229.0132 (8.40), 201.0191 (5.68), 185.0240 (1.90) | 0.75 | 0.960 | Singh et al., 2015 |
| 14. | HHDP isomer | C20H18O14 | 481.0628 | 481.0628 (100), 421.0443 (100), 300.9991 (69.36), 275.0194 (37.91), 229.0138 (8.30), 201.0188 (5.03), 185.0230 (2.20) | 0.75 | 0.960 | Singh et al., 2015 |
| 15. | digalloylglucose | C20H20O14 | 483.0748 | 483.0748 (100), 331.0676 (15.47), 313.0550 (9.74), 211.0246 (2.04), 169.0130 (82.73), 125.0229 (48.03), 151.0027 (2.15), 107.0123 (5.89) | 1.67 | 0.831 | Singh et al., 2015 |
| 16. | digalloylglucose isomer | C20H20O14 | 483.0785 | 483.0785 (100), 331.0676 (4.70), 313.0576 (13.31), 271.0459 (45.44), 211.0246 (11.17), 169.0130 (36.40), 125.0230 (32.87), 107.0122 (5.92) | 4.23 | 0.893 | Singh et al., 2015 |
| 17. | gemin D | C27H22O18 | 633.0740 | 633.0740 (100), 613.0474 (18.37), 481.0623 (3.77), 465.0675 (20.40), 445.0409 (8.60), 421.0831 (0.58), 319.0095 (4.01), 313.0565 (22.78), 300.9990 (54.64), 299.9922 (3.36), 275.0194 (7.77), 29.0134 (5.64), 245.0084 (2.45), 217.0132 (2.27), 125.0229 (23.25) | 3.73 | 1.000 | |
| 18. | pedunculaginn | C34H24O22 | 783.0676 | 783.0676 (79.16), 688.7011 (1.95), 481.0623 (2.05), 342.8359 (1.81), 300.9991 (100), 275.0196 (38.55), 229.0132 (15.14), 203.0343 (2.92), 201.187 (8.05), 185.0237 (7.04), 245.0083 (3.19), 145.0277 (2.78) | 1.85 | −1.373 | Hager et al., 2008 |
| 19. | pedunculagin isomer | C34H24O22 | 783.0696 | 783.0696 (94.52), 696.1905 (3.00), 632.2634 (2.81), 578.0688 (2.56), 419.6635 (3.15), 300.9991 (100), 275.0197 (53.08), 257.0084 (10.56), 229.0132 (15.28), 203.0343 (5.70), 201.187 (6.12), 185.0230 (2.82) | 3.28 | 1.894 | Hager et al., 2008 |
| 20. | tellimagrandin I | C34H26O22 | 785.0858 | 785.0858 (100), 492.5254 (2.38), 300.9992 (91.82), 275.0194 (40.60), 249.0403 (41.84), 229.0137 (14.38), 185.0232 (4.49), 169.0126 (13.49) | 4.15 | 1.853 | Singh et al., 2015 |
| 21. | galloyl-bis-hexahydroxyphenoyl-hexoside (casuarictin/ potentillin) | C41H28O26 | 935.0792 | 935.0792 (100), 633.0712 (2.11), 300.9989 (81.10), 229.0136 (7.32), 245.0079 (2.49), 257.0091 (4.42), 217.0138 (2.68) | 9.46 | −0.475 | Donno et al., 2013 |
| 22. | trisgalloyl HHDP glucose | C41H28O27 | 951.0797 | 951.0793 (30.06), 907.0871 (100), 847.7051 (3.06), 783.0707 (6.71), 635.1165 (3.83), 408.0540 (3.33), 341.2052 (3.05), 299.9902 (26.04), 300.9986 (85.18), 275.0205 (28.77), 245.0072 (5.83), 229.0132 (5.75), 257.0094 (5.38), 201.0178 (7.20) | 3.19 | −0.717 | Singh et al., 2015 |
| Hydroxybenzoic and hydroxycinnamic acids | |||||||
| 23. | salcylic acid | C7H6O3 | 137.0230 | 137.0229 (14.31), 93.0329 (100), 65.0381 (0.53) | 10.10 | −0.271 | RS |
| 24. | protocatechuic acid | C7H6O4 | 153.0181 | 153.0180 (15.10), 123.0438 (1.17), 109.0279 (100) | 2.04 | −7.855 | RS |
| 25. | 2,4-dihydroxybenzoic acid | C7H6O4 | 153.0181 | 153.0180 (15.10), 123.0438 (1.17), 109.0279 (100) | 3.56 | −8.770 | |
| 26. | gentisic acid | C7H6O4 | 153.0180 | 153.0180 (41.70), 123.0071 (0.23) 109.0279 (100) | 4.71 | −8.770 | RS |
| 27. | p-coumaric acid | C9H8O3 | 163.0387 | 163.0389 (87.03), 135.0436 (96.46), 119.0487 (100) | 2.955 | −8.510 | RS |
| 28. | m-coumaric acid | C9H8O3 | 163.0387 | 163.0387 (40.10), 135.0436 (30.46), 119.0487 (100) | 3.92 | −8.142 | RS |
| 29. | o-coumaric acid | C9H8O3 | 163.0390 | 163.0389 (7.89), 119.0486 (100) | 6.87 | −7.222 | RS |
| 30. | isovanillic acid | C8H8O4 | 167.0342 | 167.0342 (15.00), 152.0102 (100), 124.0147 (2.04) | 4.26 | −4.622 | |
| 31. | vanillic acid | C8H8O4 | 167.0342 | 167.0338 (100), 152.0098 (33.78), 124.0147 (11.99), 111.0070 (5.05), 95.0123 (3.62) | 7.12 | −4.921 | RS |
| 32. | caffeic acid | C9H8O4 | 179.0340 | 179.0340 (17.92), 135.0437 (100), 107.0488 (1.45) | 4.77 | −5.764 | RS |
| 33. | ferulic acid | C10H10O4 | 193.0500 | 193.0500 (8.88), 178.0263 (1.96), 149.0593 (3.56), 134.0359 (100) | 8.65 | −3.481 | RS |
| 34. | isoferulic acid | C10H10O4 | 193.0496 | 193.0496 (100), 178.0268 (6.84), 161.0231 (18.57), 149.0586 (2.18), 134.0360 (10.60) | 11.63 | −3.860 | |
| Acylquinic acids | |||||||
| 35. | 3-p-coumaroylquinic acid | C16H18O8 | 337.0925 | 337.0925 (13.04), 191.0550 (16.15), 173.0443 (3.40), 163.0387 (100) | 3.97 | 0.651 | Clifford et al., 2005 |
| 36. | 1-p-coumaroylquinic acid | C16H18O8 | 337.0936 | 337.0936 (10.27), 191.0551 (100), 173.0446 (6.66), 163.0390 (7.41) | 6.04 | 2.015 | Clifford et al., 2005 |
| 37. | 4-p-coumaroylquinic acid | C16H18O8 | 337.0931 | 337.0931 (8.57), 191.0551 (2.09), 173.0443 (100), 163.0387 (18.30) | 6.35 | 0.562 | Clifford et al., 2005 |
| 38. | 5-p-coumaroylquinic acid | C16H18O8 | 337.0918 | 337.0918 (8.80), 191.0550 (100), 163.0389 (15.75) | 7.72 | −3.236 | Clifford et al., 2005 |
| 39. | 1-caffeoylquinic acid | C16H18O9 | 353.0876 | 353.0876 (72.68), 191.0550 (100), 179.0341 (83.65), 135.0437 (77.23) | 2.08 | −0.440 | Clifford et al., 2005 |
| 40. | neochlorogenic acid | C16H18O9 | 353.0880 | 353.0881 (46.28), 191.0551 (100), 179.0339 (65.78), 173.0446 (4.13), 135.0437 (54.34) | 2.64 | 0.495 | RS |
| 41. | chlorogenic acid | C16H18O9 | 353.0870 | 353.0887 (4.75), 191.0551 (100), 179.0335 (1.68), 161.0231 (2.06), 135.0438 (1.84) | 4.34 | 2.676 | RS |
| 42. | 4-caffeoylquinic acid | C16H18O9 | 353.0880 | 353.0881 (31.42), 191.0552 (40.86), 179.0341 (65.74), 173.0444 (100), 135.0437 (50.91) | 4.84 | 0.410 | Clifford et al., 2005 |
| 43. | 3-feruloylquinic acid | C17H20O9 | 367.1041 | 367.1032 (24.57), 193.0497 (100), 173.0452 (2.42), 134.0359 (58.04) | 4.79 | 1.756 | Clifford et al., 2005 |
| 44. | 4-feruloylquinic acid | C17H20O9 | 367.1042 | 367.1036 (12.06), 173.0444 (100), 163.5176 (7.37), 134.0361 (7.81) | 6.68 | 2.083 | Clifford et al., 2005 |
| 45. | 5-feruloylquinic acid | C17H20O9 | 367.1045 | 367.1018 (17.78), 191.0551 (100), 173.0447 (8.89), 134.0358 (7.59) | 7.30 | 2.737 | Clifford et al., 2005 |
| 46. | 1,5-dicaffeoylquinic acid | C25H24O12 | 515.1214 | 515.1190 (18.05), 353.0888 (86.26), 19.0551 (100), 179.0333 (44.18), 135.0434 (37.39) | 11.42 | 3.690 | Clifford et al., 2007 |
| 47. | 3,5-dicaffeoylquinic acid | C25H24O12 | 515.1214 | 515.1217 (24.72), 353.0873 (77.33), 191.0555 (100), 179.0331 (29.88), 135.0437 (33.09) | 11.79 | 3.690 | Clifford et al., 2007 |
| 48. | 4,5-dicaffeoylquinic acid | C25H24O12 | 515.1215 | 353.0865 (39.29), 191.0551 (35.74), 179.0340 (59.64), 173.0447 (100), 135.0435 (45.53) | 12.63 | 3.923 | Clifford et al., 2007 |
| Phenylethanoid glycosides | |||||||
| 49. | protocatechuic acid O-hexoside | C13H16O9 | 315.0732 | 314.9041 (2.36), 153.0180 (100), 109.0279 (53.45) | 1.24 | 3.348 | |
| 50. | coumaroyl hexose | C15H18O8 | 325.0922 | 325.0922 (6.89), 265.0716 (100), 235.0608 (42.67), 205.0498 (67.25), 163.0388 (67.25), 145.0280 (76.94), 119.0486 (54.37) | 5.02 | −2.155 | |
| 51. | O-caffeoyl hexose | C15H18O9 | 341.0878 | 341.0878 (29.75), 281.0663 (1.28), 251.0565 (2.69), 179.0338 (34.12), 161.0231 (100), 135.0437 (15.68), 133.0280 (26.58), 119.0335 (0.68) | 3.22 | −0.016 | Clifford et al., 2007 |
| 52. | caffeic acid O-hexoside | C15H18O9 | 341.0877 | 341.0880 (20.12), 281.0666 (96.48), 251.0558 (51.86), 221.0450 (51.79), 179.0338 (100), 161.0231 (68.89), 135.0437 (65.22), 133.0280 (22.11), 119.0331 (1.41) | 3.57 | −0.367 | Clifford et al., 2007 |
| 53. | caffeic acid O-hexoside isomer | C15H18O9 | 341.0877 | 341.0878 (26.20), 281.0667 (99.55), 251.0560 (58.13), 221.0448 (58.13), 179.0339 (100), 161.0231 (61.33), 135.0438 (78.37), 133.0281 (24.21) | 4.27 | −0.191 | Clifford et al., 2007 |
| 54. | O-caffeoyldihexose | C21H28O14 | 503.1422 | 503.1417 (100), 323.0759 (4.92), 179.0339 (28.23), 161.0231 (64.90), 135.0437 (23.88), 133.0284 (16.6) | 4.51 | 3.143 | Oszmiański et al., 2015 |
| 55. | dicaffeoylhexose | C24H24O12 | 503.1220 | 503.1220 (87.04), 323.0788 (8.57), 179.0340 (100), 161.0229 (47.85), 135.0435 (87.65), 133.0283 (18.14) | 11.20 | 4.931 | |
| 56. | dicaffeoylhexose isomer | C24H24O12 | 503.1207 | 503.1204 (100), 323.0760 (12.05), 179.0341 (70.79), 161.0229 (45.51), 135.0437 (84.87), 133.0277 (11.39) | 12.69 | 3.477 | |
| Flavonoids | |||||||
| 57. | apigenin | C15H10O5 | 269.0455 | 269.0455 (100), 151.0020 (5.22), 149.0230 (6.31) | 17.92 | −0.285 | RS |
| 58. | luteolin | C15H10O6 | 285.0410 | 285.0410 (100), 241.0148 (1.07), 201.0184 (1.46), 151.0022 (1.01), 133.0280 (4.64), 107.0122 (0.39) | 15.37 | 1.785 | RS |
| 59. | chrysoeriol | C16H12O6 | 299.0558 | 299.0558 (55.66), 284.0324 (100), 255.0298 (34.04), 227.0349 (23.67) | 19.77 | −0.974 | RS |
| 60. | isorhamnetin | C16H12O7 | 315.0520 | 315.0513 (100), 300.0270 (41.20), 227.1276 (2.72), 151.0016 (2.86), 107.0123 (5.43) | 19.18 | 3.695 | RS |
| 61. | homoorientin | C21H20O11 | 447.0946 | 447.0940 (100), 357.0617 (37.28), 327.0516 (54.46), 299.0566 (10.01), 297.0417 (7.40), | 8.17 | 3.032 | RS |
| 62. | orientin | C21H20O11 | 447.0941 | 447.0941 (77.13), 357.0606 (38.10), 327.0519 (100), 297.0399 (7.35) | 8.43 | 1.801 | RS |
| 63. | kaempferol 3-O-glucoside | C21H20O11 | 447.0957 | 447.0957 (100), 285.0403 (19.61), 284.0327 (53.17), 255.0297 (47.69), 227.0344 (47.98), 211.0408 (0.93), 151.0023 (1.08), 107.0119 (1.01) | 11.42 | 5.402 | RS |
| 64. | isorhamnetin 3-O-pentoside | C21H20O11 | 447.0944 | 447.0455 (100), 366.6548 (2.12), 315.0150 (73.08), 314.0061 (17.27), 299.9912 (63.49), 285.0406 (60.99), 284.0327 (26.08), 270.9878 (15.03), 151.0026 (1.82), | 10.28 | 2.405 | de Rijke et al., 2006 |
| 65. | luteolin 7-O-glucoside | C21H20O11 | 447.0947 | 447.0947 (100), 285.0408 (56.48), 257.0452 (3.75), 151.0020 (27.49) | 11.77 | 3.233 | RS |
| 66. | isoquercitrin | C21H20O12 | 463.0888 | 463.0888 (100), 301.0356 (31.89), 300.0275 (66.48), 271.0246 (33.03), 255.0304 (11.37), 243.0298 (7.18), 227.0348 (2.34), 151.0024 (1.83) | 9.98 | 1.319 | RS |
| 67. | quercetin−7-O-hexoside | C21H20O12 | 463.0896 | 463.0896 (88.80), 301.0355 (100), 151.0023 (37.18), 107.0122 (15.76) | 13.12 | 3.025 | |
| 68. | quercetin 3-O-hexuronide | C21H18O13 | 477.0592 | 477.0592 (57.61), 301.0361 (100), 178.9989 (7.63), 151.0019 (19.23) | 9.74 | −0.116 | Oszmiański et al., 2015 |
| 69. | kaempferol 3-O-rutinoside | C27H30O15 | 593.1308 | 593.1308 (100), 285.0403 (68.74), 284.0326 (56.73), 255.0296 (34.96), 227.0345 (22.63), 107.0125 (1.80) | 2.759 | 16.43 | RS |
| 70. | rutin | C27H30O16 | 609.1484 | 609.1484 (100), 301.0356 (35.08), 300.0284 (54.53), 271.0243 (28.18), 255.0314 (7.49) | 9.60 | 4.630 | RS |
| 71. | luteolin 3-O-caffeoylhexoside | C30H26O14 | 609.1263 | 609.1263 (80.32), 285.0402 (100), 161.0233 (15.83), 135.0441 (4.12), 133.0275 (3.93), 151.0033 (3.49) | 16.12 | 2.235 | |
| Others | |||||||
| 72. | azelaic acid | C9H16O4 | 187.0968 | 187.0966 (40.44), 169.0859 (0.91), 163.5142 (0.67), 125.0957 (100), 97.0642 (8.67) | 11.83 | −1.464 | Zhao et al., 2016 |
| 73. | traumatic acid | C12H20O4 | 227.1285 | 227.1285 (10.51), 209.1178 (1.02), 183.1379 (100), 165.1273 (18.23), 81.1410 (0.53) | 20.50 | −1.639 | Sinan et al., 2020 |
| 74. | tormentic acid | C30H48O5 | 487.3419 | 487.3436 (100), 469.3323 (8.83), 425. 3414 (), 423.3272 (1.35), 379.2236 (0.33), 96.0703 (0.38) | 25.76 | −2.130 | |
| Histological Findings * | Group I—210 mg/kg | Group II—70 mg/kg | Group III—20 mg/kg |
|---|---|---|---|
| Tubulitis—outbreaks of 5–10 cells per tubular diameter | Not established | ||
| Mononuclear interstitial inflammatory infiltrate: less than 10% in female animals | 8% | 6% | 4% |
| Mononuclear interstitial inflammatory infiltrate: less than 10% in male animals | 6% | 6% | 3% |
| Glomerulitis—segmental and global in the presented glomeruli | Not established | ||
| PAC-positive hyaline thickening in more than one arteriolus | Arterial patency was reported. | ||
| Intimate arteritis with loss of luminal spaces in each arterial irrigation zone | No changes and luminal reduction in irrigation zones | ||
| Infarcts | Not established | ||
| Interstitial bleedings | Not established | ||
| Glomerulopathic changes—double contouring peripheral capillary loops in non-sclerotic glomeruli | Not established | ||
| Interstitial fibrosis—in the cortical sections | Not established | ||
| Tubular atrophy—in areas of cortical tubules | Not established | ||
| Fiber-intimal arterial thickening with lumen reduction in areas covering this indicator | Not established | ||
| Increase in mesangial matrix- in non-sclerotic glomeruli | Not established | ||
| Presentation of foamy cells | Sporadic | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, M.M.; Dimitrova, L.L.; Philipov, S.; Nikolova, I.; Vilhelmova, N.; Grozdanov, P.; Nikolova, N.; Popova, M.; Bankova, V.; Konstantinov, S.M.; et al. In Vitro Antineoplastic and Antiviral Activity and In Vivo Toxicity of Geum urbanum L. Extracts. Molecules 2022, 27, 245. https://doi.org/10.3390/molecules27010245
Zaharieva MM, Dimitrova LL, Philipov S, Nikolova I, Vilhelmova N, Grozdanov P, Nikolova N, Popova M, Bankova V, Konstantinov SM, et al. In Vitro Antineoplastic and Antiviral Activity and In Vivo Toxicity of Geum urbanum L. Extracts. Molecules. 2022; 27(1):245. https://doi.org/10.3390/molecules27010245
Chicago/Turabian StyleZaharieva, Maya M., Lyudmila L. Dimitrova, Stanislav Philipov, Ivanka Nikolova, Neli Vilhelmova, Petar Grozdanov, Nadya Nikolova, Milena Popova, Vassya Bankova, Spiro M. Konstantinov, and et al. 2022. "In Vitro Antineoplastic and Antiviral Activity and In Vivo Toxicity of Geum urbanum L. Extracts" Molecules 27, no. 1: 245. https://doi.org/10.3390/molecules27010245
APA StyleZaharieva, M. M., Dimitrova, L. L., Philipov, S., Nikolova, I., Vilhelmova, N., Grozdanov, P., Nikolova, N., Popova, M., Bankova, V., Konstantinov, S. M., Zheleva-Dimitrova, D., & Najdenski, H. M. (2022). In Vitro Antineoplastic and Antiviral Activity and In Vivo Toxicity of Geum urbanum L. Extracts. Molecules, 27(1), 245. https://doi.org/10.3390/molecules27010245