Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Copolyesters
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. 1H and 13C Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Thermal Analysis
2.5. Mechanical Analysis and Tensile Properties
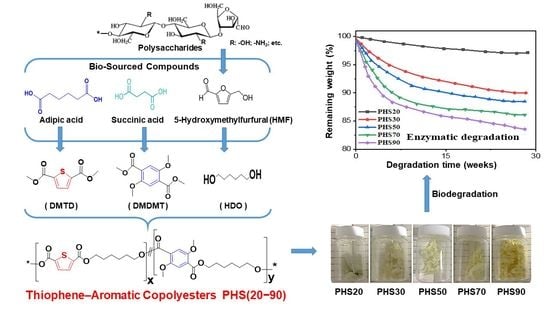
2.6. Degradation of Copolyesters PHS(20–90)
3. Experimental Section
3.1. Materials
3.2. Synthesis of Copolyesters PHS(20–90)
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Namazi, H. Polymers in our daily life. BioImpacts 2017, 7, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Tamo, A.K.; Doench, I.; Walter, L.; Montembault, A.; Sudre, G.; David, L.; Morales-Helguera, A.; Selig, M.; Rolauffs, B.; Bernstein, A.; et al. Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues. Polymers 2021, 13, 1663. [Google Scholar] [CrossRef] [PubMed]
- Lall, A.; Tamo, A.K.; Doench, I.; David, L.; De Oliveira, P.N.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Bravo, S.; Doench, I.; Molina, P.; Bentley, F.E.; Tamo, A.K.; Passieux, R.; Lossada, F.; David, L.; Osorio-Madrazo, A. Functional Bionanocomposite Fibers of Chitosan Filled with Cellulose Nanofibers Obtained by Gel Spinning. Polymers 2021, 13, 1563. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Japu, C.; Alla, A.; de Ilarduya, A.M.; García-Martín, M.G.; Benito, E.; Galbis, J.A.; Muñoz-Guerra, S. Bio-based aromatic copolyesters made from 1,6-hexanediol and bicyclic diacetalized d-glucitol. Polym. Chem. 2012, 3, 2092. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Pham, N.T.-H.; Nguyen, V.-T. Morphological and Mechanical Properties of Poly (Butylene Terephthalate)/High-Density Poly-ethylene Blends. Adv. Mater. Sci. Eng. 2020, 2020, 8890551. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Abushammala, H.; Pontes, J.F.; Gomes, G.H.M.; Osorio-Madrazo, A.; Thiré, R.; Pereira, F.; Laborie, M.-P.G. Swelling, viscoelastic, and anatomical studies on ionic liquid-swollen Norway spruce as a screening tool toward ionosolv pulping. Holzforschung 2015, 69, 1059–1067. [Google Scholar] [CrossRef]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Novel preparation route for cellulose nanowhiskers. In Abstracts of Papers of the American Chemical Society; American Chemical Society: New Orleans, LA, USA, 2013. [Google Scholar]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine micro-structure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Eder, M.; Rueggeberg, M.; Pandey, J.K.; Harrington, M.J.; Nishiyama, Y.; Putaux, J.-L.; Rochas, C.; Burgert, I. Reorientation of Cellulose Nanowhiskers in Agarose Hydrogels under Tensile Loading. Biomacromolecules 2012, 13, 850–856. [Google Scholar] [CrossRef]
- Saviello, D.; Cespi, D.; Sharma, V.; Miao, S.; Cucciniello, R. The Frontier of Biobased Polymers: Synthesis, Characterization, Ap-plication, and Sustainability Assessment. Int. J. Polym. Sci. 2017, 2017, 5638598. [Google Scholar] [CrossRef] [Green Version]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO 2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amine, S.; Montembault, A.; Fumagalli, M.; Osorio-Madrazo, A.; David, L. Controlled Polyelectrolyte Association of Chitosan and Carboxylated Nano-Fibrillated Cellulose by Desalting. Polymers 2021, 13, 2023. [Google Scholar] [CrossRef]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; De Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio-Madrazo, A.; David, L.; Montembault, A.; Viguier, E.; Cachon, T. Hydrogel Composites Comprising Chitosan and Cellulose Nanofibers. International Publication No. WO 2019/175279 A1, 19 September 2019. U.S. Publication No. 2021/0047479, 18 February 2021. [Google Scholar]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Osorio-Madrazo, A.; Laborie, M.-P. Hydroxypropyl tannin derivatives from Pinus pinaster (Ait.) bark. Ind. Crop. Prod. 2013, 49, 730–739. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, T.A.; Osorio-Madrazo, A.; Laborie, M.-P.G. Synthesis and physicochemical properties of hy-droxypropyl tannins from maritime pine bark (Pinus pinaster Ait.). Holzforschung 2014, 68, 411–418. [Google Scholar] [CrossRef]
- Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials 2017, 10, 400. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Covas, C.A.P.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Von Palubitzki, L.; Wang, Y.; Hoffmann, S.; Vidal-Y-Sy, S.; Zobiak, B.; Failla, A.V.; Schmage, P.; John, A.; Osorio-Madrazo, A.; Bauer, A.T.; et al. Differences of the tumour cell glycocalyx affect binding of capsaicin-loaded chitosan nanocapsules. Sci. Rep. 2020, 10, 22443. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Makwana, V.A.; Larrañaga, A.; Vilas, J.L.; Shaver, M.P. Thermal, structural and degradation properties of an ar-omatic–aliphatic polyester built through ring-opening polymerization. Polym. Chem. 2017, 8, 3530–3538. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman, A.M.; Schmidt-D’Aloisio, K.; Dong, Y.; Springborg, M. Properties of polythiophene and related conjugated polymers: A density-functional study. Phys. Chem. Chem. Phys. 2005, 7, 2714–2722. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Amna, B.; Siddiqi, H.M.; Hassan, A.; Ozturk, T. Recent developments in the synthesis of regioregular thiophene-based conjugated polymers for electronic and optoelectronic applications using nickel and palladium-based catalytic systems. RSC Adv. 2020, 10, 4322–4396. [Google Scholar] [CrossRef] [Green Version]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulopoulou, N.; Pipertzis, A.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Green polymeric materials: On the dynamic homogeneity and miscibility of furan-based polyester blends. Polymers 2019, 174, 187–199. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characteri-zation of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Wang, G.; Yang, G.; Jiang, M.; Wang, R.; Liang, Y.; Zhou, G. Poly(propylene naphthalate-co-propylene 2,5-thiophenedicarboxylate)s derived from bio-based 2,5-thiophenedicarboxylic acid (TDCA): Synthesis and properties. Polym. Test. 2021, 93, 106955. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.; Jiang, M.; Zhang, Q.; Wang, R.; Wang, H.; Zhou, G. High Tg and tough poly(butylene 2,5-thiophenedicarboxylate- co -1,4-cyclohexanedimethylene 2,5-thiophenedicarboxylate)s: Synthesis and characterization. J. Appl. Polym. Sci. 2020, 137, 48634. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Liang, Q.; Zhou, G. New bio-based copolyesters poly(trimethylene 2,5-thiophenedicarboxylate-co-trimethylene terephthalate): Synthesis, crystallization behavior, thermal and mechanical properties. Polymers 2019, 173, 27–33. [Google Scholar] [CrossRef]
- Desrosiers, N.; Bergeron, J.-Y.; Belletête, M.; Durocher, G.; Leclerc, M. Synthesis and characterization of novel aromatic polyesters derived from thiophenes. Polymers 1996, 37, 675–680. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Turan, H.T.; Yavuz, I.; Aviyente, V. Understanding the Impact of Thiophene/Furan Substitution on Intrinsic Charge-Carrier Mobility. J. Phys. Chem. C 2017, 121, 25682–25690. [Google Scholar] [CrossRef]
- Pellis, A.; Weinberger, S.; Gigli, M.; Guebitz, G.M.; Farmer, T.J. Enzymatic synthesis of biobased polyesters utilizing aromatic diols as the rigid component. Eur. Polym. J. 2020, 130, 109680. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhang, X.-Q.; Shen, A.; Zhu, J.; Song, P.-A.; Wang, H.; Liu, X.-Q. Synthesis and Properties Investigation of Thio-phene-aromatic Polyesters: Potential Alternatives for the 2,5-Furandicarboxylic Acid-based Ones. Chin. J. Polym. Sci. 2020, 38, 1082–1091. [Google Scholar] [CrossRef]
- Le Marquer, N.; Laurent, M.; Martel, A. A Practical and Cost-Effective Method for the Synthesis of Bicy-clo[2.2.2]octane-1,4-dicarboxylic Acid. Synthesis 2015, 47, 2185–2187. [Google Scholar]
- Wang, G.; Liang, Y.; Jiang, M.; Zhang, Q.; Wang, R.; Wang, H.; Zhou, G. Synthesis and characterization of bio-based polyesters from 2,5-thiophenedicarboxylic acid. Polym. Degrad. Stab. 2019, 168, 108942. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Cheng, Z.; Siegu, W.M.K.; Jing, X.; Chen, J.; Adom, E.K.; Muaz, A.; Gauthier, M. High performance sulfur-containing copolyesters from bio-sourced aromatic monomers. Express Polym. Lett. 2022, 16, 102–114. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct Synthesis of 1,6-Hexanediol from HMF over a Heterogeneous Pd/ZrP Catalyst using Formic Acid as Hydrogen Source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Wang, F.; Li, R.; Yu, X.; Kang, L.; Zhao, J.; Li, A. One-pot biosynthesis of 1,6-hexanediol from cyclohexane by de novo designed cascade biocatalysis. Green Chem. 2020, 22, 7476–7483. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules 2020, 25, 541. [Google Scholar] [CrossRef] [Green Version]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Durand, G.; le Foll, M.D.; Trombotto, S. Water-Soluble 2,5-Anhydro-d-mannofuranose Chain End Chitosan Oligomers of a Very Low Molecular Weight: Synthesis and Characterization. Biomacromolecules 2019, 20, 4353–4360. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Smidsrød, O. Preparation and characterisation of oligosaccharides produced by nitrous acid depolymerisation of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Tamo, A.K.; Doench, I.; Helguera, A.M.; Hoenders, D.; Walther, A.; Osorio Madrazo, A. Biodegradation of Crystalline Cellulose Nanofibers by Means of Enzyme Immobilized-Alginate Beads and Microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Samyn, P.; Osorio-Madrazo, A. Native Crystalline Polysaccharide Nanofibers: Processing and Properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–36. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Rychter, P.; Kawalec, M.; Sobota, M.; Kurcok, P.; Kowalczuk, M. Study of Aliphatic-Aromatic Copolyester Degradation in Sandy Soil and Its Ecotoxicological Impact. Biomacromolecules 2010, 11, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Boukis, A.C.; Llevot, A.; Meier, M.A.R. High Glass Transition Temperature Renewable Polymers via Biginelli Multicomponent Polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef]
- Kanasty, R.L.; Vegas, A.J.; Ceo, L.M.; Maier, M.; Charisse, K.; Nair, J.K.; Langer, R.; Anderson, D.G. Sequence-Defined Oligomers from Hydroxyproline Building Blocks for Parallel Synthesis Applications. Angew. Chem. Int. Ed. 2016, 55, 9529–9533. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, W.; Walter, S.E.G.; Beckers, M.; Seide, G.H.; Gries, T. Thermal Analysis of Phase Transitions and Crystallization in Polymeric Fibers. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; IntechOpen: London, UK, 2013. [Google Scholar]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Balderrama, C.I.P.; Martínez, I.C.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Collins, C.R.; Ribelli, T.G.; Matyjaszewski, K.; Gordon, G.J.; Kowalewski, T.; Yaron, D.J. Tuning the molecular weight dis-tribution from atom transfer radical polymerization using deep reinforcement learning. Mol. Syst. Des. Eng. 2018, 3, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Madrazo, A.; Laborie, M.-P. Morphological and Thermal Investigations of Cellulosic Bionanocomposites. Biopolym. Nanocompos. 2013, 411–436. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(propylene 2,5-thiophenedicarboxylate) vs. Poly(propylene 2,5-furandicarboxylate): Two Examples of High Gas Barrier Bio-Based Polyesters. Polymers 2018, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Madhu, G.; Bhunia, H.; Bajpai, P.K.; Chaudhary, V. Mechanical and morphological properties of high density polyethylene and polylactide blends. J. Polym. Eng. 2014, 34, 813–821. [Google Scholar] [CrossRef]
- Sahraeian, R.; Esfandeh, M. Mechanical and morphological properties of LDPE/perlite nanocomposite films. Polym. Bull. 2017, 74, 1327–1341. [Google Scholar] [CrossRef]
- Wu, H.; Lv, S.; He, Y.; Qu, J.-P. The study of the thermomechanical degradation and mechanical properties of PET recycled by industrial-scale elongational processing. Polym. Test. 2019, 77, 105882. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Kasuya, K.-I. Biodegradability of poly(3-hydroxyalkanoate) and poly(ε-caprolactone) via biological carbon cycles in marine environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Koitabashi, M.; Noguchi, M.T.; Sameshima-Yamashita, Y.; Hiradate, S.; Suzuki, K.; Yoshida, S.; Watanabe, T.; Shinozaki, Y.; Tsushima, S.; Kitamoto, H.K. Degradation of biodegradable plastic mulch films in soil environment by phylloplane fungi isolated from gramineous plants. AMB Express 2012, 2, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Sample | |||||
|---|---|---|---|---|---|
| PHS20 | PHS30 | PHS50 | PHS70 | PHS90 | |
| DMTD:DMDMT molar ratio | 0.2:0.8 | 0.3:0.7 | 0.5:0.5 | 0.7 0.3 | 0.9:0.1 |
| Yield (%) | 92 | 94 | 95 | 91 | 93 |
| 1st Step (°C) | 160 | 160 | 170 | 180 | 180 |
| Clearing point (h) | 3 | 2.5 | 2 | 1.5 | 1 |
| Mn (g/mol) | 15,000 | 18,500 | 15,500 | 14,500 | 12,300 |
| Mw (g/mol) | 30,100 | 35,700 | 38,800 | 29,500 | 27,500 |
| Polydispersity index (Đ) | 2.00 | 2.01 | 2.50 | 2.11 | 2.31 |
| Ha (mol %) | 35.3 | 44.3 | 50.8 | 59.4 | 63.1 |
| Hb (mol %) | 64.7 | 55.7 | 49.2 | 40.6 | 36.9 |
| Wavenumber [cm−1]. | Characteristic Group |
|---|---|
| 3120 | thiophene |
| 2927, 2855 | -CH2- from aliphatic chains |
| 1720 | -C(O)- from ester groups |
| 1270 | -C-(O)-O-C- from ester groups (sp2) |
| 1078 | -C-C-O- from ester groups (sp3) |
| Label | Chemical Shift [ppm] | Group |
|---|---|---|
| a | 7.77–7.80 | TH-H |
| b | 7.55–7.62 | Ar-H |
| c | 3.81–3.88 | -O-CH3 |
| d, e | 4.30–4.43 | TH-O-CH2-CH2-O-Ar |
| f | 1.79 | -O-CH2-CH2 |
| g | 1.45 | -O-CH2-CH2-CH2- |
| Copolyesters | Td, 5% [°C] | Td, 50% [°C] | Td, max [°C] | Tm [°C] | Tg [°C] | ∆Hc [J/g] | R700 [wt %] |
|---|---|---|---|---|---|---|---|
| PHS20 | 284 | 395 | 410 | 175 | 85 | −55.6 | 6.9 |
| PHS30 | 319 | 409 | 412 | 166 | 89 | −56.4 | 5.8 |
| PHS50 | 331 | 419 | 426 | 183 | 100 | −56.8 | 3.5 |
| PHS70 | 280 | 392 | 406 | 163 | 82 | −51.2 | 5.7 |
| PHS90 | 277 | 387 | 395 | 155 | 70 | −50.4 | 4.7 |
| Sample | Tensile Modulus [MPa] | Tensile Strength [MPa] | Elongation at Break [%] |
|---|---|---|---|
| PET [45,47] | 1137 | 60 | 5.04 |
| HDPE [45] | 1670 ± 54 | 52.70 ± 3.7 | 188 ± 19 |
| LDPE [46] | 364 ± 4 | 14.9 ± 2 | 69 ± 4 |
| PHS20 | 1185 ± 12 | 37 ± 2 | 327 ± 9 |
| PHS30 | 1273 ± 12 | 42 ± 2 | 335± 9 |
| PHS50 | 1552 ± 12 | 80 ± 2 | 370 ± 9 |
| PHS70 | 895 ± 12 | 27 ± 2 | 298 ± 9 |
| PHS90 | 836 ± 12 | 23 ± 2 | 282 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djouonkep, L.D.W.; Tamo, A.K.; Doench, I.; Selabi, N.B.S.; Ilunga, E.M.; Lenwoue, A.R.K.; Gauthier, M.; Cheng, Z.; Osorio-Madrazo, A. Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules 2022, 27, 325. https://doi.org/10.3390/molecules27010325
Djouonkep LDW, Tamo AK, Doench I, Selabi NBS, Ilunga EM, Lenwoue ARK, Gauthier M, Cheng Z, Osorio-Madrazo A. Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules. 2022; 27(1):325. https://doi.org/10.3390/molecules27010325
Chicago/Turabian StyleDjouonkep, Lesly Dasilva Wandji, Arnaud Kamdem Tamo, Ingo Doench, Naomie Beolle Songwe Selabi, Emmanuel Monga Ilunga, Arnaud Regis Kamgue Lenwoue, Mario Gauthier, Zhengzai Cheng, and Anayancy Osorio-Madrazo. 2022. "Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies" Molecules 27, no. 1: 325. https://doi.org/10.3390/molecules27010325
APA StyleDjouonkep, L. D. W., Tamo, A. K., Doench, I., Selabi, N. B. S., Ilunga, E. M., Lenwoue, A. R. K., Gauthier, M., Cheng, Z., & Osorio-Madrazo, A. (2022). Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules, 27(1), 325. https://doi.org/10.3390/molecules27010325