Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection
Abstract
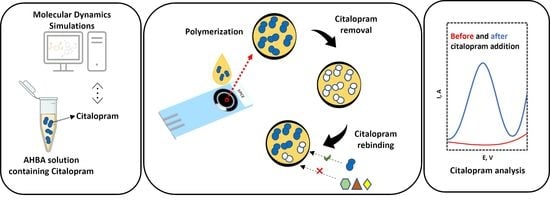
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus and Equipment
2.3. Fabrication of the MIP-CTL Sensor and Electrochemical Analysis
2.4. Computational Setup
3. Results and Discussion
3.1. MD Simulations
3.1.1. Density and Self-Diffusion Coefficients
3.1.2. Local Structure Analysis: RDFs and H-Bonds
3.2. Electrochemical Preparation of MIP-CTL Sensor and Its Recognition Abilities
3.3. Optimization of Experimental Parameters in Preparation and Detection Process
3.4. Electrochemical Characterizations of the Stepwise MIP-CTL Construction
3.5. Analytical Performance
3.6. Selectivity and Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- OECD. Health at a Glance 2017: OECD Indicators; OECD Publication: Paris, France, 2017. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Z.; Wang, J.; Ye, Z.; Zhang, L.; Niu, J. Photodegradation of three antidepressants in natural waters: Important roles of dissolved organic matter and nitrate. Sci. Total Environ. 2022, 802, 149825. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical compounds in aquatic environments— occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vázquez, F.M.; Delerue-Matos, C. Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere 2020, 239, 124729. [Google Scholar] [CrossRef]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- Schmidtová, Z.; Kodešová, R.; Grabicová, K.; Kočárek, M.; Fér, M.; Švecová, H.; Klement, A.; Nikodem, A.; Grabic, R. Competitive and synergic sorption of carbamazepine, citalopram, clindamycin, fexofenadine, irbesartan and sulfamethoxazole in seven soils. J. Contam. Hydrol. 2020, 234, 103680. [Google Scholar] [CrossRef] [PubMed]
- OECD. Health at a Glance 2021: OECD Indicators; OECD Publication: Paris, France, 2021. [Google Scholar] [CrossRef]
- Fernandes, S.; Sosa-Napolskij, M.; Lobo, G.; Silva, I. Impact of the COVID-19 pandemic in the Portuguese population: Consumption of alcohol, stimulant drinks, illegal substances, and pharmaceuticals. PLoS ONE 2021, 16, e0260322. [Google Scholar] [CrossRef]
- Argaluza, J.; Domingo-echaburu, S.; Orive, G.; Medrano, J.; Hernandez, R.; Lertxundi, U. Environmental pollution with psychiatric drugs. World J. Psychiatry 2021, 11, 791–804. [Google Scholar] [CrossRef]
- Ziegler, M.; Knoll, S.; Köhler, H.R.; Tisler, S.; Huhn, C.; Zwiener, C.; Triebskorn, R. Impact of the antidepressant citalopram on the behaviour of two different life stages of brown trout. PeerJ 2020, 2020, e8765. [Google Scholar] [CrossRef]
- Bezchlibnyk-Butler, K.; Aleksic, I.; Kennedy, S.H. Citalopram—A review of pharmacological and clinical effects. J. Psychiatry Neurosci. 2000, 25, 241–254. [Google Scholar]
- Budău, M.; Hancu, G.; Rusu, A.; Cârcu-Dobrin, M.; Muntean, D.L. Chirality of modern antidepressants: An overview. Adv. Pharm. Bull. 2017, 7, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Madej, M.; Matoga, D.; Skaźnik, K.; Porada, R.; Baś, B.; Kochana, J. A voltammetric sensor based on mixed proton-electron conducting composite including metal-organic framework JUK-2 for determination of citalopram. Microchim. Acta 2021, 188, 184. [Google Scholar] [CrossRef] [PubMed]
- Berzas Nevado, J.J.; Guiberteau Cabanillas, C.; Villaseñor Llerena, M.J.; Rodríguez Robledo, V. Enantiomeric determination, validation and robustness studies of racemic citalopram in pharmaceutical formulations by capillary electrophoresis. J. Chromatogr. A 2005, 1072, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Vasskog, T.; Berger, U.; Samuelsen, P.J.; Kallenborn, R.; Jensen, E. Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J. Chromatogr. A 2006, 1115, 187–195. [Google Scholar] [CrossRef]
- Gołyszny, M.; Obuchowicz, E. Are neuropeptides relevant for the mechanism of action of SSRIs? Neuropeptides 2019, 75, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, E.A.; Bazovkina, D.V.; Evsyukova, V.S.; Kulikov, A.V. Acute Administration of Imipramine and Citalopram Increases Activity of Striatal-Enriched Tyrosine Protein Phosphatase (STEP) in Brain of Zebrafish Danio rerio. Bull. Exp. Biol. Med. 2021, 170, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.J.; Reisinger, L.S.; Richmond, E.K.; Rosi, E.J. Exposure to a common antidepressant alters crayfish behavior and has potential subsequent ecosystem impacts. Ecosphere 2021, 12, e03527. [Google Scholar] [CrossRef]
- Staszny, A.; Dobosy, P.; Maasz, G.; Szalai, Z.; Jakab, G.; Pirger, Z.; Szeberenyi, J.; Molnar, E.; Pap, L.O.; Juhasz, V.; et al. Effects of pharmaceutically active compounds (PhACs) on fish body and scale shape in natural waters. PeerJ 2021, 9, e10642. [Google Scholar] [CrossRef]
- Ma, L.-D.; Li, J.; Li, J.-J.; Liu, M.; Yan, D.-Z.; Shi, W.-Y.; Xu, G. Occurrence and source analysis of selected antidepressants and their metabolites in municipal wastewater and receiving surface water. Environ. Sci. Process. Impacts 2018, 20, 1020–1029. [Google Scholar] [CrossRef]
- Peng, Y.; Gautam, L.; Hall, S.W. The detection of drugs of abuse and pharmaceuticals in drinking water using solid-phase extraction and liquid chromatography-mass spectrometry. Chemosphere 2019, 223, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Sarıkaya, M.; Ulusoy, H.I.; Morgul, U.; Ulusoy, S.; Tartaglia, A.; Yılmaz, E.; Soylak, M.; Locatelli, M.; Kabir, A. Sensitive determination of Fluoxetine and Citalopram antidepressants in urine and wastewater samples by liquid chromatography coupled with photodiode array detector. J. Chromatogr. A 2021, 1648, 462215. [Google Scholar] [CrossRef]
- Nouws, H.; Delerue-Matos, C.; Barros, A. Electrochemical determination of citalopram by adsorptive stripping voltammetry-determination in pharmaceutical products. Anal. Lett. 2006, 39, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Nouws, H.P.A.; Delerue-Matos, C.; Barros, A.A.; Maesen, E.; Moreira, S.C.P.A.; Neves, M.M.P.S. Static and hydrodynamic monitoring of citalopram based on its electro-oxidation behavior at a glassy-carbon surface. Anal. Lett. 2008, 41, 2171–2185. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Dhanjai; Sharma, S. Bismuth (III) oxide/glassy carbon sensor for sensing of antidepressant drug escitalopram in micellar media. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 178–184. [Google Scholar] [CrossRef]
- Ghaedi, H.; Afkhami, A.; Madrakian, T.; Soltani-Felehgari, F. Construction of novel sensitive electrochemical sensor for electro-oxidation and determination of citalopram based on zinc oxide nanoparticles and multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 59, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Akbari, A. A novel voltammetric sensor for citalopram based on multiwall carbon nanotube/(poly(p-aminobenzene sulfonic acid)/β-cyclodextrin). Mater. Sci. Eng. C 2016, 62, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Keypour, H.; Saremi, S.G.; Veisi, H.; Noroozi, M. Electrochemical determination of citalopram on new Schiff base functionalized magnetic Fe3O4 nanoparticle/MWCNTs modified glassy carbon electrode. J. Electroanal. Chem. 2016, 780, 160–168. [Google Scholar] [CrossRef]
- Daneshvar, L.; Rounaghi, G.H.; Es’haghi, Z.; Chamsaz, M.; Tarahomi, S. Fabrication a new modified electrochemical sensor based on Au–Pd bimetallic nanoparticle decorated graphene for citalopram determination. Mater. Sci. Eng. C 2016, 69, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; Kochana, J.; Baś, B. Selective and Highly Sensitive Voltammetric Determination of Citalopram with Glassy Carbon Electrode. J. Electrochem. Soc. 2019, 166, H359–H369. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Cui, B.; Liu, P.; Liu, X.; Liu, S.; Zhang, Z. Molecularly imprinted polymers for electrochemical detection and analysis: Progress and perspectives. J. Mater. Res. Technol. 2020, 9, 12568–12584. [Google Scholar] [CrossRef]
- Mostafiz, B.; Bigdeli, S.A.; Banan, K.; Afsharara, H.; Hatamabadi, D.; Mousavi, P.; Hussain, C.M.; Keçili, R.; Ghorbani-Bidkorbeh, F. Molecularly imprinted polymer-carbon paste electrode (MIP-CPE)-based sensors for the sensitive detection of organic and inorganic environmental pollutants: A review. Trends Environ. Anal. Chem. 2021, 32, e00144. [Google Scholar] [CrossRef]
- Moreira Gonçalves, L. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Akpan, E.D.; Adekunle, A.S.; Fayemi, O.E.; Sherif, E.M.; Ebenso, E.E. Molecularly imprinted polymers ( MIPs ) based electrochemical sensors for the determination of catecholamine neurotransmitters—Review. Electrochem. Sci. Adv. 2021, 1, e2000026. [Google Scholar] [CrossRef]
- Seguro, I.; Pacheco, J.G.; Delerue-Matos, C. Low cost, easy to prepare and disposable electrochemical molecularly imprinted sensor for diclofenac detection. Sensors 2021, 21, 1975. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Cordeiro, M.N.D.S.; Melo, A.; Delerue-Matos, C. Azithromycin electrochemical detection using a molecularly imprinted polymer prepared on a disposable screen-printed electrode. Anal. Methods 2020, 12, 1486–1494. [Google Scholar] [CrossRef]
- Del Sole, R.; Mele, G.; Bloise, E.; Mergola, L. Green Aspects in Molecularly Imprinted Polymers by Biomass Waste Utilization. Polymers 2021, 13, 2430. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Viveiros, R.; Rebocho, S.; Casimiro, T. Green strategies for molecularly imprinted polymer development. Polymers 2018, 10, 306. [Google Scholar] [CrossRef] [Green Version]
- Cowen, T.; Karim, K.; Piletsky, S. Computational approaches in the design of synthetic receptors—A review. Anal. Chim. Acta 2016, 936, 62–74. [Google Scholar] [CrossRef]
- Suryana, S.; Mutakin; Rosandi, Y.; Hasanah, A.N. An update on molecularly imprinted polymer design through a computational approach to produce molecular recognition material with enhanced analytical performance. Molecules 2021, 26, 1891. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Rational development of molecular imprinted carbon paste electrode for Furazolidone detection: Theoretical and experimental approach. Sens. Actuators B Chem. 2021, 329, 129112. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Development of a molecular imprinted electrochemiluminescence sensor for amitriptyline detection: From MD simulations to experimental implementation. Electrochim. Acta 2021, 397, 139273. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. A simple electrochemical detection of atorvastatin based on disposable screen-printed carbon electrodes modified by molecularly imprinted polymer: Experiment and simulation. Anal. Chim. Acta 2022, 1194, 339410. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, I.A.; Golker, K.; Olsson, G.D.; Suriyanarayanan, S.; Wiklander, J.G. The use of computational methods for the development of molecularly imprinted polymers. Polymers 2021, 13, 2841. [Google Scholar] [CrossRef]
- James, M.; Murtola, T.; Schulz, R.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, M.; Ramsey, S.; Kurtzman, T.; Cho, A.E. Water Pharmacophore: Designing Ligands using Molecular Dynamics Simulations with Water. Sci. Rep. 2018, 8, 10400. [Google Scholar] [CrossRef]
- Salo-ahen, O.M.H.; Alanko, I.; Bhadane, R.; Bonvin, A.M.J.J.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-kakkonen, M.; Larsen, A.S.; et al. and Pharmaceutical Development. Processes 2021, 9, 71. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water liquid water. J. Chem. Phys. 1998, 79, 926–935. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Software News and Update Packmol: A Package for Building Initial Configurations. J. Comput. Chem. 2009, 30, 2158–2163. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distribution. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrinelo, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. Quiet High-Resolution Computer Models of a Plasma. J. Comput. Phys. 1974, 14, 148–158. [Google Scholar] [CrossRef]
- Voroshylova, I.V.; Ferreira, E.S.C.; Malček, M.; Costa, R.; Pereira, C.M.; Cordeiro, M.N.D.S. Influence of the anion on the properties of ionic liquid mixtures: A molecular dynamics study. Phys. Chem. Chem. Phys. 2018, 20, 14899–14918. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, N.M.; Voroshylova, I.V.; Koverga, V.A.; Ferreira, E.S.C.; Cordeiro, M.N.D.S. Influence of alcohols on the inter-ion interactions in ionic liquids: A molecular dynamics study. J. Mol. Liq. 2019, 294, 111538. [Google Scholar] [CrossRef]
- Ferreira, E.S.C.; Voroshylova, I.V.; Koverga, V.A.; Pereira, C.M.; Cordeiro, M.N.D.S. New Force Field Model for Propylene Glycol: Insight to Local Structure and Dynamics. J. Phys. Chem. B 2017, 121, 10906–10921. [Google Scholar] [CrossRef]
- Voroshylova, I.V.; Ferreira, E.S.C.; Koverga, V.A.; Pereira, C.M.; Cordeiro, M.N.D.S. Structure and noncovalent interactions in ionic liquids mixtures and deep eutectic solvents. In Theoretical and Computational Approaches to Predicting Ionic Liquid Properties; Mathew, A.S.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–157. [Google Scholar]
- Nilsson, M.E.S.; Suriyanarayanan, S.; Kathiravan, S.; Yli-Kauhaluoma, J.; Kotiaho, T.; Nicholls, I.A. Enantioselective hyperporous molecularly imprinted thin film polymers. RSC Adv. 2019, 9, 33653–33656. [Google Scholar] [CrossRef] [Green Version]
- Luzar, A.; Chandler, D. Hydrogen-bond kinetics in liquid water. Lett. Nat. 1996, 379, 55–57. [Google Scholar] [CrossRef]
- Kan, K.; Yamamoto, H.; Kaneko, D.; Tateyama, S.; Kaneko, T. Novel π-conjugated bio-based polymer, poly(3-amino-4-hydroxybenzoic acid),and its solvatochromism. Pure Appl. Chem. 2014, 86, 685–690. [Google Scholar] [CrossRef]
- Giraud, L.; Grelier, S.; Grau, E.; Hadziioannou, G.; Brochon, C.; Cramail, H.; Cloutet, E. Upgrading the chemistry of π-conjugated polymers toward more sustainable materials. J. Mater. Chem. C 2020, 8, 9792–9810. [Google Scholar] [CrossRef]








| Number of Molecules | ||||||
|---|---|---|---|---|---|---|
| Mixture | Molar Ratio (CTL:AHBA) | S(+)-CTL | R(−)-CTL | AHBA | H2O | Total Number of Interaction Sites |
| 1 | 1:1 | 25 | 25 | 50 | 10,000 | 33,150 |
| 2 | 1:2 | 25 | 25 | 100 | 10,000 | 34,050 |
| 3 | 1:3 | 25 | 25 | 150 | 10,000 | 34,950 |
| 4 | 1:4 | 25 | 25 | 200 | 10,000 | 35,850 |
| 5 | 1:6 | 25 | 25 | 300 | 10,000 | 37,650 |
| D × 109, m2 s−1 | ||||
|---|---|---|---|---|
| Mixture | d, kg m−3 | CTL | AHBA | H2O |
| 1 | 1009.43 | 0.13 ± 0.02 | 0.28 ± 0.01 | 5.21 ± 0.11 |
| 2 | 1021.88 | 0.11 ± 0.00 | 0.20 ± 0.02 | 4.87 ± 0.10 |
| 3 | 1032.86 | 0.12 ± 0.01 | 0.24 ± 0.02 | 4.53 ± 0.06 |
| 4 | 1043.96 | 0.03 ± 0.04 | 0.13 ± 0.00 | 4.32 ± 0.04 |
| 5 | 1064.54 | 0.06 ± 0.01 | 0.16 ± 0.03 | 3.94 ± 0.04 |
| Mixture | Total H-Bonds | Interactions Per 50 CTL Molecules |
|---|---|---|
| 1 | 8.96 | 9 |
| 2 | 43.58 | 22 |
| 3 | 67.45 | 22 |
| 4 | 70.22 | 18 |
| 5 | 78.54 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Seguro, I.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection. Molecules 2022, 27, 3315. https://doi.org/10.3390/molecules27103315
Rebelo P, Pacheco JG, Voroshylova IV, Seguro I, Cordeiro MNDS, Delerue-Matos C. Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection. Molecules. 2022; 27(10):3315. https://doi.org/10.3390/molecules27103315
Chicago/Turabian StyleRebelo, Patrícia, João G. Pacheco, Iuliia V. Voroshylova, Isabel Seguro, Maria Natália D. S. Cordeiro, and Cristina Delerue-Matos. 2022. "Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection" Molecules 27, no. 10: 3315. https://doi.org/10.3390/molecules27103315
APA StyleRebelo, P., Pacheco, J. G., Voroshylova, I. V., Seguro, I., Cordeiro, M. N. D. S., & Delerue-Matos, C. (2022). Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection. Molecules, 27(10), 3315. https://doi.org/10.3390/molecules27103315