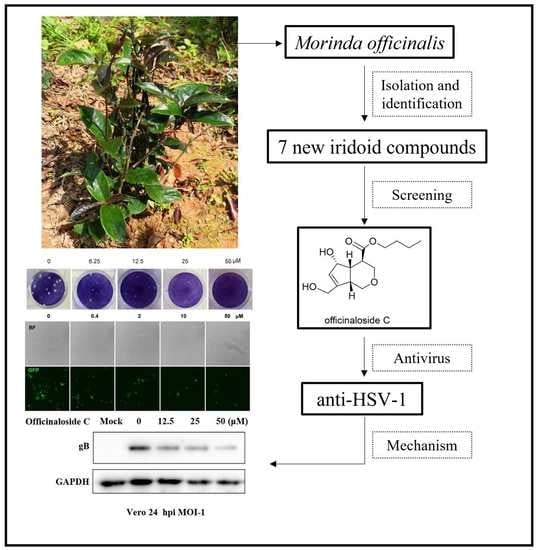
Antiviral Activities of Officinaloside C against Herpes Simplex Virus-1
Abstract
:1. Introduction
2. Results
2.1. Chemical Compounds from MO
2.2. Antiviral Activity and Cytotoxicity Evaluation of Officinaloside C
2.3. Officinaloside C Can Inhibit Virus Plaque Formation
2.4. Officinaloside C Has Inhibitory Effects on Gene Expression after HSV-1 Infection
2.5. Officinaloside C Can Inhibit Virus Replication
2.6. Officinaloside C WB Results Analysis
3. Materials and Methods
3.1. Plan Material and Isolation of New Iridoid Compounds of Officinaloside A–G
3.2. Cells and Viruses
3.3. Cytotoxicity Assay
3.4. Antiviral Assay
3.5. Fluorescence Analysis
3.6. Western Blot
3.7. Real-Time Fluorescent Quantitative PCR (RT-PCR)
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. Globally, an Estimated Two-Thirds of the Population under 50 Are Infected with Herpes Simplex Virus Type 1. Available online: https://apps.who.int/mediacentre/news/releases/2015/herpes/en/index.html (accessed on 1 March 2022).
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Puma, D.D.L.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J. Herpes simplex encephalitis: Adolescents and adults. Antivir. Res. 2006, 71, 141–148. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- Coen, D.M.; Schaffer, P.A. Antiherpesvirus drugs: A promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2003, 2, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Piret, J.; Boivin, G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: Diagnosis and management. Curr. Opin. Infect. Dis. 2016, 29, 654–662. [Google Scholar] [CrossRef]
- Chen, J.L.; Blanc, P.; Stoddart, C.A.; Bogan, M.; Rozhon, E.J.; Parkinson, N.; Ye, Z.; Cooper, R.; Balick, M.; Nanakorn, A.W.; et al. New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. J. Nat. Prod. 1998, 61, 1298. [Google Scholar] [CrossRef]
- Zhang, H.; Rothwang, K.; Mesecar, A.D.; Sabahi, A.; Rong, L.; Fong, H.H.S. Lamiridosins, hepatitis C virus entry inhibitors from Lamium album. J. Nat. Prod. 2009, 72, 2158–2162. [Google Scholar] [CrossRef]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino K-i Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Antiinflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 2015. [Google Scholar]
- Editorial Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Liu, M.; Cai, M.; Ding, P. Oligosaccharides from traditional Chinese herbal medicines: A review of chemical diversity and biological activities. Am. J. Chin. Med. 2021, 49, 577–608. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, M.; Chen, P.; Liu, H.; Wang, Y.; Yang, D.; Zhao, Z.; Ding, P. Iridoids with anti-inflammatory effect from the aerial parts of Morinda officinalis How. Fitoterapia 2021, 153, 104991. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Galhardi, L.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef]
- Hilterbrand, A.T.; Daly, R.E.; Heldwein, E.E. Contributions of the Four Essential Entry Glycoproteins to HSV-1 Tropism and the Selection of Entry Routes. mBio 2021, 12, e00143-21. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Su, G.; Wang, Y.; Wang, Z.; Jia, J.; Qing, S.; Huang, L.; Wang, Y.; Zheng, K.; et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses 2019, 11, 466. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.H.; Xin, H.L.; Xu, Y.M.; Shen, Y.; He, Y.Q.; Yeh, H.; Lin, B.; Song, H.T.; Liu, J.; Yang, H.Y.; et al. Morinda officinalis How.—A comprehensive review of traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2018, 213, 230–255. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019, 169, 185–199. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Indian Morinda species: A review. Phytother. Res. 2019, 34, 924–1007. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive Natural Antivirals: An Updated Review of the Available Plants and Isolated Molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Crovella, S. Blue Laser Light Counteracts HSV-1 in the SH-SY5Y Neuronal Cell Model of Infection. Life 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Gerriets, V. Acyclovir, StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]




| Compounds | 100 μM | 10 μM | 1 μM | Conclusion |
|---|---|---|---|---|
| Officinaloside A | ++++ | ++++ | ++++ | × |
| Officinaloside B | ++++ | ++++ | ++++ | × |
| Officinaloside C | + | + | ++ | √ |
| Officinaloside D | ++++ | ++++ | ++++ | × |
| Officinaloside E | ++++ | ++++ | ++++ | × |
| Officinaloside F | ++++ | ++++ | ++++ | × |
| Officinaloside G | ++++ | ++++ | ++++ | × |
| ACV | + | + | + | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Cai, M.; Wang, Y.; Ding, P. Antiviral Activities of Officinaloside C against Herpes Simplex Virus-1. Molecules 2022, 27, 3365. https://doi.org/10.3390/molecules27113365
Xiao J, Cai M, Wang Y, Ding P. Antiviral Activities of Officinaloside C against Herpes Simplex Virus-1. Molecules. 2022; 27(11):3365. https://doi.org/10.3390/molecules27113365
Chicago/Turabian StyleXiao, Ji, Miaomiao Cai, Yifei Wang, and Ping Ding. 2022. "Antiviral Activities of Officinaloside C against Herpes Simplex Virus-1" Molecules 27, no. 11: 3365. https://doi.org/10.3390/molecules27113365
APA StyleXiao, J., Cai, M., Wang, Y., & Ding, P. (2022). Antiviral Activities of Officinaloside C against Herpes Simplex Virus-1. Molecules, 27(11), 3365. https://doi.org/10.3390/molecules27113365