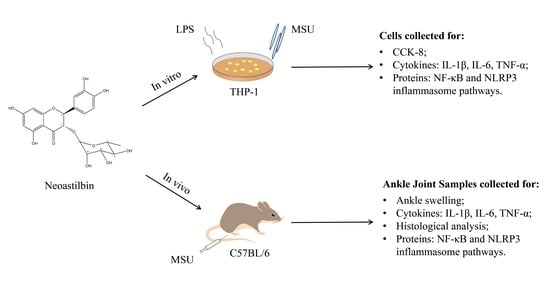
The Protective Effects of Neoastilbin on Monosodium Urate Stimulated THP-1-Derived Macrophages and Gouty Arthritis in Mice through NF-κB and NLRP3 Inflammasome Pathways
Abstract
:1. Introduction
2. Results
2.1. Effect of Neoastilbin on Cell Viability in THP-1-Derived Macrophages
2.2. Effect of Neoastilbin on the Secretion of Inflammatory Cytokines in THP-1-Derived Macrophages
2.3. Effects of Neoastilbin on Protein Expression of NF-κB and NLRP3 Inflammasome Pathways in THP-1-Derived Macrophages
2.4. Effect of Neoastilbin on MSU-Induced Ankle Swelling in GA Mice
2.5. Effect of Neoastilbin on the Levels of Inflammatory Cytokines in GA Mice
2.6. Effect of Neoastilbin on MSU-Induced Inflammatory Infiltration in Ankle Joints
2.7. Effects of Neoastilbin on Protein Expression of NF-κB and NLRP3 Inflammasome Pathways in GA mice
3. Discussion
4. Materials and Methods
4.1. Samples and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay by CCK-8
4.4. Inflammatory Cytokine Determination in THP-1-Derived Macrophages by ELISA Kits
4.5. Analysis of Protein Expression of NF-κB and NLRP3 Inflammasome Pathways in THP-1-Derived Macrophages
4.6. Animals
4.7. Measurement of Ankle Swelling in GA Mice
4.8. Inflammatory Cytokine Determination in GA Mice by ELISA Kits
4.9. Analysis of MSU-Induced Inflammatory Infiltration in Ankle Joints
4.10. Analysis of Protein Expression of NF-κB and NLRP3 Inflammasome Pathways in GA Mice
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desai, J.; Steiger, S.; Anders, H.J. Molecular pathophysiology of gout. Trends Mol. Med. 2017, 23, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, B.; Wang, J.; Ma, B.; Zhang, W. Pyolysin of trueperella pyogenes induces pyroptosis and IL-1beta release in murine macrophages through Potassium/NLRP3/Caspase-1/Gasdermin D pathway. Front. Immunol. 2022, 13, 832458. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Zhang, S.; Ruan, Z.; Liu, X.; Yang, G.; Jia, Y.; Li, Y.; Pan, P.; Wang, W.; Li, G.; et al. AP-1 signaling pathway promotes pro-IL-1beta transcription to facilitate NLRP3 inflammasome activation upon influenza A virus infection. Virulence 2022, 13, 502–513. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Alehashemi, S.; Goldbach-Mansky, R. Human autoinflammatory diseases mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-inflammasome dysregulation updates on diagnosis, treatment, and the respective roles of IL-1 and IL-18. Front. Immunol. 2020, 11, 1840. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, J.; Zhang, Y.; Liu, Y.; Ma, C.; Yi, F.; Zheng, Y.; Zhang, L.; Chen, T.; Liu, H.; et al. USP5 attenuates NLRP3 inflammasome activation by promoting autophagic degradation of NLRP3. Autophagy 2021, 1–15. [Google Scholar] [CrossRef]
- Wang, B.; Bhattacharya, M.; Roy, S.; Tian, Y.; Yin, Q. Immunobiology and structural biology of AIM2 inflammasome. Mol. Asp. Med. 2020, 76, 100869. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome complexes: Emerging mechanisms and effector functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Gong, T.; Jiang, W.; Zhou, R. GPCRs in NLRP3 inflammasome activation, regulation, and therapeutics. Trends Pharmacol. Sci. 2018, 39, 798–811. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; von Moltke, J.; Jones, J.W.; Vance, R.E.; Monack, D.M. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 2010, 8, 471–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1beta activation on macrophages. Ann. Rheum Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.P.; Gladue, H.S.; Singh, M.K.; FitzGerald, J.D.; Bae, S.; Prakash, S.; Kaldas, M.; Gogia, M.; Berrocal, V.; Townsend, W.; et al. Treatment of acute gout: A systematic review. Semin. Arthritis Rheum 2014, 44, 31–38. [Google Scholar] [CrossRef]
- van Durme, C.M.; Wechalekar, M.D.; Landewe, R.B.; Pardo Pardo, J.; Cyril, S.; van der Heijde, D.; Buchbinder, R. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2021, 12, CD010120. [Google Scholar]
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2018, 57, i4–i11. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, N. The safety of treatment options available for gout. Expert Opin. Drug Saf. 2017, 16, 429–436. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, H.; Niu, Z.; Li, J.; Pan, G.; Tian, H.; Wei, L.; Chen, L.; Yang, P.; Wang, J.; et al. Acute oral colchicine caused gastric mucosal injury and disturbance of associated microbiota in mice. Toxicology 2021, 461, 152908. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.; Shi, Y.; Zhang, X.; Tian, C.; Xia, D. Antioxidant and anti-Inflammatory activities of six flavonoids from Smilax glabra Roxb. Molecules 2020, 25, 5295. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020, 158, 104894. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yan, Z.; Shui, X.; Qi, W.; Chen, Y.; Xu, X.; Hu, Y.; Guo, W.; Shang, P. Astilbin prevents osteoarthritis development through the TLR4/MD-2 pathway. J. Cell Mol. Med. 2020, 24, 13104–13114. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, Y.; Ji, J.; Zhang, S.; Yang, X.; Xu, J.; Wang, J.S.; Chen, Z.; Xia, B.; Shen, H.; et al. Astilbin from Smilax glabra Roxb. alleviates high-fat diet-induced metabolic dysfunction. Food Funct. 2022, 13, 5023–5036. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The national health and nutrition examination survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.C.; Taylor, W.J.; Dalbeth, N. An observational study of gout prevalence and quality of care in a national Australian general practice population. J. Rheumatol. 2015, 42, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kwak, S.G.; Lee, H.; Kim, S.K.; Choe, J.Y.; Park, S.H. Prevalence and incidence of gout in Korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017, 37, 1499–1506. [Google Scholar] [CrossRef]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef] [Green Version]
- Ozen, S.; Kone-Paut, I.; Gul, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum 2017, 47, 115–120. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Kluck, V.; Liu, R.; Joosten, L.A.B. The role of interleukin-1 family members in hyperuricemia and gout. Joint Bone Spine 2021, 88, 105092. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Kida, H.; Iwai, A. Cell cytotoxity and anti-glycation activity of taxifolin-rich extract from Japanese larch, Larix kaempferi. Heliyon 2019, 5, e02047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of Naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.P.; Carmody, R.J. NF-kappaB and the transcriptional control of inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [PubMed]
- Jiang, R.H.; Xu, J.J.; Zhu, D.C.; Li, J.F.; Zhang, C.X.; Lin, N.; Gao, W.Y. Glycyrrhizin inhibits osteoarthritis development through suppressing the PI3K/AKT/NF-kappaB signaling pathway in vivo and in vitro. Food Funct. 2020, 11, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Yue, Y.; Wang, X.; Li, H.; Yan, S. Luteolin relieved DSS-induced colitis in mice via HMGB1-TLR-NF-kappaB signaling pathway. Inflammation 2021, 44, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, W.; Mu, H.; Qin, T.; Long, F.; Ren, L.; Tang, H.; Liu, J.; Zeng, M. HSP60 regulates monosodium urate crystal-induced inflammation by activating the TLR4-NF-kappaB-MyD88 signaling pathway and disrupting mitochondrial function. Oxid. Med. Cell Longev. 2020, 2020, 8706898. [Google Scholar] [CrossRef]
- Lee, H.E.; Yang, G.; Park, Y.B.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Epigallocatechin-3-Gallate prevents acute gout by suppressing NLRP3 inflammasome activation and mitochondrial DNA synthesis. Molecules 2019, 24, 2138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Deng, G.; Huang, B.; Kai, G.; Chen, K.; Li, J. A purified biflavonoid extract from Selaginella moellendorffii alleviates gout arthritis via NLRP3/ASC/Caspase-1 Axis suppression. Front. Pharmacol. 2021, 12, 676297. [Google Scholar] [CrossRef]
- ElSayed, S.; Jay, G.D.; Cabezas, R.; Qadri, M.; Schmidt, T.A.; Elsaid, K.A. Recombinant human proteoglycan 4 regulates phagocytic activation of monocytes and reduces IL-1beta secretion by urate crystal stimulated gout PBMCs. Front. Immunol. 2021, 12, 771677. [Google Scholar] [CrossRef]
- Pan, Y.G.; Huang, M.T.; Sekar, P.; Huang, D.Y.; Lin, W.W.; Hsieh, S.L. Decoy Receptor 3 inhibits monosodium urate-induced NLRP3 inflammasome activation via reduction of reactive oxygen species production and lysosomal rupture. Front. Immunol. 2021, 12, 638676. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal. Transduct. Target Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Choi, N.; Yang, G.; Jang, J.H.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Loganin alleviates gout inflammation by suppressing NLRP3 inflammasome activation and mitochondrial damage. Molecules 2021, 26, 1071. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of porcine circovirus 2 and pseudorabies virus enhances immunosuppression and inflammation through NF-kappaB, JAK/STAT, MAPK, and NLRP3 pathways. Int. J. Mol. Sci. 2022, 23, 4469. [Google Scholar] [CrossRef]
- Adachi, S.I.; Sasaki, K.; Kondo, S.; Komatsu, W.; Yoshizawa, F.; Isoda, H.; Yagasaki, K. Antihyperuricemic effect of urolithin A in cultured hepatocytes and model mice. Molecules 2020, 25, 5136. [Google Scholar] [CrossRef]











| Group | Dosage/mg·kg−1 | 2 h | 4 h | 6 h | 10 h | 24 h |
|---|---|---|---|---|---|---|
| Control | - | 20.35 ± 5.19 | 34.90 ± 4.75 | 32.89 ± 14.48 | 18.61 ± 9.61 | 15.25 ± 6.86 |
| MSU | - | 29.81 ± 9.14 # | 50.05 ± 12.29 ## | 66.84 ± 15.34 ## | 55.93 ± 16.91 ## | 54.26 ± 16.22 ## |
| MSU +Colchicine | 1 | 32.25 ± 8.72 | 45.68 ± 7.62 | 49.17 ± 10.65 * | 36.23 ± 13.21 * | 20.64 ± 16.21 ** |
| MSU +Neoastilbin | 25 | 27.58 ± 11.54 | 49.75 ± 9.01 | 56.59 ± 6.77 | 50.44 ± 14.71 | 43.93 ± 8.17 |
| 50 | 40.25 ± 10.08 | 47.58 ± 8.62 | 55.42 ± 10.43 | 38.43 ± 10.74 * | 27.96 ± 7.49 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, F.; Zhang, X.; Wu, C.; Wang, Y.; Yao, Y.; Xia, D. The Protective Effects of Neoastilbin on Monosodium Urate Stimulated THP-1-Derived Macrophages and Gouty Arthritis in Mice through NF-κB and NLRP3 Inflammasome Pathways. Molecules 2022, 27, 3477. https://doi.org/10.3390/molecules27113477
Xu W, Li F, Zhang X, Wu C, Wang Y, Yao Y, Xia D. The Protective Effects of Neoastilbin on Monosodium Urate Stimulated THP-1-Derived Macrophages and Gouty Arthritis in Mice through NF-κB and NLRP3 Inflammasome Pathways. Molecules. 2022; 27(11):3477. https://doi.org/10.3390/molecules27113477
Chicago/Turabian StyleXu, Wenjing, Fenfen Li, Xiaoxi Zhang, Chenxi Wu, Yan Wang, Yanjing Yao, and Daozong Xia. 2022. "The Protective Effects of Neoastilbin on Monosodium Urate Stimulated THP-1-Derived Macrophages and Gouty Arthritis in Mice through NF-κB and NLRP3 Inflammasome Pathways" Molecules 27, no. 11: 3477. https://doi.org/10.3390/molecules27113477
APA StyleXu, W., Li, F., Zhang, X., Wu, C., Wang, Y., Yao, Y., & Xia, D. (2022). The Protective Effects of Neoastilbin on Monosodium Urate Stimulated THP-1-Derived Macrophages and Gouty Arthritis in Mice through NF-κB and NLRP3 Inflammasome Pathways. Molecules, 27(11), 3477. https://doi.org/10.3390/molecules27113477