Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach
Abstract
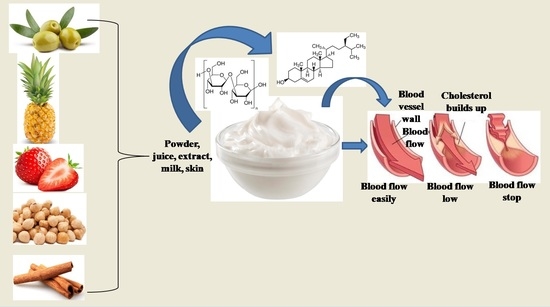
:1. Introduction
2. Hypercholesterolemia
2.1. Low-Density Lipoprotein-Cholesterol and the Concept of Hypercholesterolemia
2.2. Epidemiology
2.3. Investigation and Laboratory Assessment
2.4. Treatment
2.4.1. Non-Pharmacological Therapy
2.4.2. Pharmacological Therapy
HMG-CoA Reductase Inhibitors (Atorvastatin, Lovastatin, Simvastatin, Fluvastatin, Rosuvastatin, Pravastatin)
Bile Acid Resins (Colesevelam, Colestipol, Cholestyramine)
Fibric Acids (Gemfibrozil, Fenofibrate, Clofibrate) and Niacin
Ezetimibe
2.4.3. Herbal Treatment
3. Yogurt
3.1. Concept, History, and Health Effects
3.2. Concept of Food Enrichment
3.3. Phyto-Enrichment of Yogurt
4. Phyto-Enriched Yogurt and Hypercholesterolemia Management: Clinical Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Verschuren, W.M.; Jacobs, D.R.; Bloemberg, B.P.; Kromhout, D.; Menotti, A.; Aravanis, C.; Toshima, H. Serum total cholesterol and long-term coronary heart disease mortality in different cultures: Twenty-five-year follow-up of the seven countries study. JAMA 1995, 274, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.; Daviglus, M.L.; Garside, D.B.; Dyer, A.R.; Greenland, P.; Neaton, J.D. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000, 284, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [PubMed]
- Guallar-Castillón, P.; Gil-Montero, M.; León-Muñoz, L.M.; Graciani, A.; Bayán-Bravo, A.; Taboada, J.M.; Rodríguez-Artalejo, F. Magnitude and management of hypercholesterolemia in the adult population of Spain, 2008–2010: The ENRICA Study. Rev. Esp. Cardiol. 2012, 65, 551–558. [Google Scholar] [CrossRef]
- Catapano, A.L.; Reiner, Ž.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Wood, D. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011, 217, 1–44. [Google Scholar] [CrossRef]
- Kuulasmaa, K.; Tunstall-Pedoe, H.; Dobson, A.; Fortmann, S.; Sans, S.; Tolonen, H.; Ferrario, M. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet 2000, 355, 675–687. [Google Scholar] [CrossRef]
- Flores-Mateo, G.; Grau, M.; O’Flaherty, M.; Ramos, R.; Elosua, R.; Violan-Fors, C.; Capewell, S. Analyzing the coronary heart disease mortality decline in a Mediterranean population: Spain 1988-2005. Rev. Esp. Cardiol. 2011, 64, 988–996. [Google Scholar] [CrossRef]
- Rodŕiguez-Artalejo, F.; Guallar-Castillon, P.; Banegas, J.R. Critical review and proposals for improvement of the health information systems on cardiovascular diseases in Spain. Med. Clin. 2008, 131, 302–311. [Google Scholar]
- Supiyev, A.; Nurgozhin, T.; Zhumadilov, Z.; Peasey, A.; Hubacek, J.A.; Bobak, M. Prevalence, awareness, treatment and control of dyslipidemia in older persons in urban and rural population in the Astana region, Kazakhstan. BMC Public Health 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Gu, D.; Reynolds, K.; Wu, X.; Muntner, P.; Zhao, J.; Whelton, P.K. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation 2004, 110, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Carroll, M.D.; Lacher, D.A.; Sorlie, P.D.; Cleeman, J.I.; Gordon, D.J.; Wolz, M.; Johnson, C.L. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA 2005, 294, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primatesta, P.; Poulter, N.R. Levels of dyslipidaemia and improvement in its management in England: Results from the Health Survey for England 2003. Clin. Endocrinol. 2006, 64, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.D.; Lacher, D.A.; Wolz, M.; Sorlie, P.D. 30-year trends in serum lipids among United States adults: Results from the national health and nutrition examination surveys II, III, and 1999–2006. Am. J. Cardiol. 2011, 107, 1868–1870. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: Prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol—United States, 1999–2002 and 2005–2008. MMWR Morb. Mortal. Wkly Rep. 2011, 60, 109–114. [Google Scholar]
- Roth, G.A.; Fihn, S.D.; Mokdad, A.H.; Aekplakorn, W.; Hasegawa, T.; Lim, S.S. High total serum cholesterol, medication coverage and therapeutic control: An analysis of national health examination survey data from eight countries. Bull. World Health Organ. 2011, 89, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Yadav, H. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902717. [Google Scholar] [CrossRef] [Green Version]
- Anandharaj, M.; Sivasankari, B.; Parveen Rani, R. Effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: A review. Chin. J. Biol. 2014, 572754, 1–7. [Google Scholar] [CrossRef]
- Proestos, C. Superfoods: Recent data on their role in the prevention of diseases. Curr. Res. Nutr. Food Sci. J. 2018, 6, 576–593. [Google Scholar] [CrossRef]
- van den Driessche, J.J.; Plat, J.; Mensink, R.P. Effects of superfoods on risk factors of metabolic syndrome: A systematic review of human intervention trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef]
- Astrup, A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. Am. J. Clin. Nutr. 2014, 99, 1235S–1242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.A.; Panahi, S.; Daniel, N.; Tremblay, A.; Marette, A. Yogurt and cardiometabolic diseases: A critical review of potential mechanisms. Adv. Nutr. 2017, 8, 812–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [PubMed]
- Wang, H.; Livingston, K.A.; Fox, C.S.; Meigs, J.B.; Jacques, P.F. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr. Res. 2013, 33, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, K.; Yu, L.G.; Yang, J.R.; Zhang, X.Y.; Zhou, H.; Shi, Y.J.; Qin, L.Q. The association between milk consumption and the metabolic syndrome: A cross-sectional study of the residents of Suzhou, China and a meta-analysis. Br. J. Nutr. 2020, 123, 1013–1023. [Google Scholar] [CrossRef]
- Cormier, H.; Thifault, É.; Garneau, V.; Tremblay, A.; Drapeau, V.; Pérusse, L.; Vohl, M.C. Association between yogurt consumption, dietary patterns, and cardio-metabolic risk factors. Eur. J. Nutr. 2016, 55, 577–587. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; De Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Buendia, J.R.; Li, Y.; Hu, F.B.; Cabral, H.J.; Bradlee, M.L.; Quatromoni, P.A.; Moore, L.L. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am. J. Hypertens 2018, 31, 557–565. [Google Scholar] [CrossRef]
- Watanabe, D.; Kuranuki, S.; Sunto, A.; Matsumoto, N.; Nakamura, T. Daily yogurt consumption improves glucose metabolism and insulin sensitivity in young nondiabetic Japanese subjects with type-2 diabetes risk alleles. Nutrients 2018, 10, 1834. [Google Scholar] [CrossRef] [Green Version]
- Bridge, A.; Brown, J.; Snider, H.; Nasato, M.; Ward, W.E.; Roy, B.D.; Josse, A.R. Greek yogurt and 12 weeks of exercise training on strength, muscle thickness and body composition in lean, untrained, university-aged males. Front. Nutr. 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, S.; Gallant, A.; Tremblay, A.; Pérusse, L.; Després, J.P.; Drapeau, V. The relationship between yogurt consumption, body weight, and metabolic profiles in youth with a familial predisposition to obesity. Eur. J. Clin. Nutr. 2019, 73, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Paththinige, C.S.; Sirisena, N.D.; Dissanayake, V.H.W. Genetic determinants of inherited susceptibility to hypercholesterolemia–a comprehensive literature review. Lipids Health Dis. 2017, 16, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Soutar, A.K.; Naoumova, R.P. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 214–225. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Soran, H.; Durrington, P.N. Hypercholesterolaemia and its management. BMJ 2008, 337, a993. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Rao, R.S.; Misra, A.; Sharma, S.K. Recent trends in epidemiology of dyslipidemias in India. Indian Heart J. 2017, 69, 382–392. [Google Scholar] [CrossRef]
- Reddy, K.S.; Prabhakaran, D.; Chaturvedi, V.; Jeemon, P.; Thankappan, K.R.; Ramakrishnan, L.; Jaison, T.M. Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bull. World Health Organ. 2006, 84, 461–469. [Google Scholar] [CrossRef]
- Kinra, S.; Bowen, L.J.; Lyngdoh, T.; Prabhakaran, D.; Reddy, K.S.; Ramakrishnan, L.; Ebrahim, S. Sociodemographic patterning of non-communicable disease risk factors in rural India: A cross sectional study. BMJ 2010, 341, c4974. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Mathur, P. Surveillance of cardiovascular disease risk factors in India: The need & scope. Indian J. Med. Res. 2010, 132, 634–642. [Google Scholar]
- Pandey, R.M.; Gupta, R.; Misra, A.; Misra, P.; Singh, V.; Agrawal, A.; Sharma, V. Determinants of urban–rural differences in cardiovascular risk factors in middle-aged women in India: A cross-sectional study. Int. J. Cardio. 2013, 163, 157–162. [Google Scholar] [CrossRef]
- Guptha, S.; Gupta, R.; Deedwania, P.; Bhansali, A.; Maheshwari, A.; Gupta, A.; Sharma, K.K. Cholesterol lipoproteins and prevalence of dyslipidemias in urban Asian Indians: A cross sectional study. Indian Heart J. 2014, 66, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.R.; Anjana, R.M.; Deepa, M.; Pradeepa, R.; Bhansali, A.; Dhandania, V.K.; ICMR–INDIAB Collaborative Study Group. Prevalence of dyslipidemia in urban and rural India: The ICMR–INDIAB study. PLoS ONE 2014, 9, e96808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zahrani, J.; Shubair, M.M.; Al-Ghamdi, S.; Alrasheed, A.A.; Alduraywish, A.A.; Alreshidi, F.S.; Aldossari, K.K. The prevalence of hypercholesterolemia and associated risk factors in Al-Kharj population, Saudi Arabia: A cross-sectional survey. BMC Cardiovas. Disord. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Blacher, J.; Gabet, A.; Vallée, A.; Ferrières, J.; Bruckert, E.; Farnier, M.; Olié, V. Prevalence and management of hypercholesterolemia in France, the Esteban observational study. Medicine 2020, 99, e23445. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulos, E.; Panagiotakos, D.B.; Polystipioti, A. Diet, lifestyle factors and hypercholesterolemia in elderly men and women from Cyprus. Lipids Health Dis. 2005, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shawar, S.M.; Al-Bati, N.A.; Al-Mahameed, A.; Nagalla, D.S.; Obeidat, M. Hypercholesterolemia among apparently healthy university students. Oman Med. J. 2012, 27, 274. [Google Scholar] [CrossRef] [PubMed]
- Khonputsa, P.; Veerman, J.L.; Vos, T.; Aekplakorn, W.; Bertram, M.; Abbott-Klafter, J.; Lim, S.S. Joint prevalence and control of hypercholesterolemia and hypertension in Thailand: Third national health examination survey. Asia Pac. J. Public Health 2012, 24, 185–194. [Google Scholar] [CrossRef]
- Posadas-Romero, C.; Tapia-Conyer, R.; Lerman-Garber, I.; Zamora-González, J.; Cardoso-Saldaña, G.; Salvatierra-Izaba, B.; Sepúlveda-Amor, J.A. Cholesterol levels and prevalence of hypercholesterolemia in a Mexican adult population. Atherosclerosis 1995, 118, 275–284. [Google Scholar] [CrossRef]
- Aryan, Z.; Mahmoudi, N.; Sheidaei, A.; Rezaei, S.; Mahmoudi, Z.; Gohari, K.; Farzadfar, F. The prevalence, awareness, and treatment of lipid abnormalities in Iranian adults: Surveillance of risk factors of non-communicable diseases in Iran 2016. J. Clin. Lipidol. 2018, 12, 1471–1481. [Google Scholar] [CrossRef]
- Caroll, M.D.; Cheryl, D.F.; Duong, T.N. Total and High-density Lipoprotein Cholesterol in Adults: United States, 2015–2016. 2017. Available online: https://www.cdc.gov/nchs/data/databriefs/db290.pdf. (accessed on 12 December 2020).
- Fujiyoshi, N.; Arima, H.; Satoh, A.; Ojima, T.; Nishi, N.; Okuda, N.; NIPPON DATA 2010 Research Group. Associations between socioeconomic status and the prevalence and treatment of hypercholesterolemia in a general Japanese population: NIPPON DATA2010. J. Atheroscler. Thromb. 2018, 25, 606–620. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.M.; Choudhury, S.R.; Ahmed, J.; Talukder, M.H.; Rahman, A.S. Blood glucose and cholesterol levels in adult population of Bangladesh: Results from STEPS 2006 survey. Indian Heart J. 2016, 68, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifin, H.M.; Lourdes, T.G.R.; Ab Majid, N.L.; Abd Hamid, H.A.; Hasani, W.S.R.; Ling, M.Y.; Omar, M.A. Hypercholesterolemia prevalence, awareness, treatment and control among adults in Malaysia: The 2015 National Health and Morbidity Survey, Malaysia. Glob. J. Health Sci. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Olusi, S.O.; Prabha, K.; Sulaiman, A.; Sugathan, T.N. Prevalence of hypercholesterolemia in a Kuwaiti hospital outpatient population. Ann. Saudi. Med. 1997, 17, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Morgan, J.; Siddiq, S.; Mackness, M.I.; Miller, J.P.; Durrington, P.N. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolemia. BMJ 2000, 321, 1497. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.E.D.S.; França, C.N.; Correr, C.J.; Zucker, M.L.; Andriolo, A.; Scartezini, M. Clinical correlation between a point-of-care testing system and laboratory automation for lipid profile. Clin. Chim. Acta 2015, 446, 263–266. [Google Scholar] [CrossRef]
- Owen, W.E.; Thatcher, M.L.; Crabtree, K.J.; Greer, R.W.; Strathmann, F.G.; Straseski, J.A.; Genzen, J.R. Body fluid matrix evaluation on a Roche cobas 8000 system. Clin. Biochem. 2015, 48, 911–914. [Google Scholar] [CrossRef]
- Singh, A.; Dubey, A.; Sonker, A.; Chaudhary, R. Evaluation of various methods of point-of care testing of haemoglobin concentration in blood donors. Blood Transfus. 2014, 29, 1–7. [Google Scholar]
- Matteucci, E.; Della Bartola, L.; Rossi, L.; Pellegrini, G.; Giampietro, O. Improving CardioCheck PA analytical performance: Three-year study. Clin. Chem. Lab. Med. 2014, 52, 1291–12966. [Google Scholar] [CrossRef]
- Gupta, M.; Vandana, S.; Sidharth, M. Hyperlipidemia: An Updated Review. Int. J. Biopharm. Toxicol. Res. 2011, 1, 81–89. [Google Scholar]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W. Dietary fiber, lipids and atherosclerosis. Am. J. Cardiol. 1987, 60, 17–22. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S.; Smith, J. Advanced Nutrition and Human Metabolism, 6th ed.; Wadsworth Publishing: Belmont, CA, USA, 2013. [Google Scholar]
- Nie, Y.; Luo, F. Dietary fiber: An opportunity for a global control of hyperlipidemia. Oxid. Med. Cell Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef]
- Andersson, M.; Ellegård, L.; Andersson, H. Oat bran stimulates bile acid synthesis within 8 h as measured by 7α-hydroxy-4-cholesten-3-one. Am. J. Clin. Nutr. 2002, 76, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Dhaliya, S.A.; Surya, A.S.; Dawn, V.T.; Betty, C.; Arun, K.; Sunil, C. A review of hyperlipidemia and medicinal plants. Int. JA PS BMS 2013, 2, 219–237. [Google Scholar]
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Aznar, L.A.M.; Ral, P.C.; Ortega Anta, R.M.; Díaz Martín, J.J.; Baladia, E.; Basulto, J.; Salas-Salvado, J. Scientific evidence about the role of yogurt and other fermented milks in the healthy diet for the spanish population. Nutr. Hosp. 2013, 28, 2039–2089. [Google Scholar]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Marette, A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr. Rev. 2018, 76, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Weerathilake, W.A.D.V.; Rasika, D.M.D.; Ruwanmali, J.K.U.; Munasinghe, M.A.D.D. The evolution, processing, varieties and health benefits of yogurt. Int. J. Sci. Res. Publ. 2014, 4, 1–10. [Google Scholar]
- United States Department of Agriculture (USDA). Yogurt. 2020. Available online: https://reedir.arsnet.usda.gov/codesearchwebapp/(S(v04bt44vdd5jhdz0lbv5s1he))/codesearch.aspx (accessed on 18 December 2020).
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Sharifi-Rad, J.; Verma, R.; Machado, M.; Kumar, D.; Cruz-Martins, N. Edible mushrooms’ enrichment in food and feed: A mini review. Int. J. Food Sci. Tech. 2022, 57, 1386–1398. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Marques, M.P.M.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [Green Version]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E–knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostertag, L.M.; O’Kennedy, N.; Kroon, P.A.; Duthie, G.G.; De Roos, B. Impact of dietary polyphenols on human platelet function–a critical review of controlled dietary intervention studies. Mol. Nutr. Food Res. 2010, 54, 60–81. [Google Scholar] [CrossRef] [PubMed]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br. J. Nutr. 2001, 85, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coïsson, J.D.; Travaglia, F.; Piana, G.; Capasso, M.; Arlorio, M. Euterpe oleracea juice as a functional pigment for yogurt. Food Res. Int. 2005, 38, 893–897. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J. Food Sci. 2008, 73, C241–C248. [Google Scholar] [CrossRef]
- Blando, F.; Gerardi, C.; Nicoletti, I. Sour cherry (Prunus cerasus L.) anthocyanins as ingredients for functional foods. J. Biomed. Biotechnol. 2004, 2004, 253. [Google Scholar] [CrossRef] [Green Version]
- Gläßgen, W.E.; Wray, V.; Strack, D.; Metzger, J.W.; Seitz, H.U. Anthocyanins from cell suspension cultures of Daucus carota. Phytochemistry 1992, 31, 1593–1601. [Google Scholar] [CrossRef]
- Hiroyuki, H.; Kousuke, H.; Eiji, N.; Mariko, O.; Yoshihito, K.; Setsuro, H.; Takeshi, K. Enhanced anthocyanin production from grape callus in an air-lift type bioreactor using a viscous additive-supplemented medium. J. Biosci. Bioeng. 2002, 94, 135–139. [Google Scholar] [CrossRef]
- Curtin, C.; Zhang, W.; Franco, C. Manipulating anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol. Lett. 2003, 25, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Plata, N.; Konczak-Islam, I.; Jayram, S.; McClelland, K.; Woolford, T.; Franks, P. Effect of methyl jasmonate and p-coumaric acid on anthocyanin composition in a sweet potato cell suspension culture. Biochem. Eng. J. 2003, 14, 171–177. [Google Scholar] [CrossRef]
- O’connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of pineapple waste powder on probiotic growth, antioxidant and antimutagenic activities of yogurt. J. Food Sci. Technol. 2016, 53, 1698–1708. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Shokery, E.S.; El-Ziney, M.G.; Yossef, A.H.; Mashaly, R.I. Effect of green tea and Moringa leave extracts fortification on the physicochemical, rheological, sensory and antioxidant properties of set-type yoghurt. Adv. Dairy Res. 2017, 5, 2. [Google Scholar] [CrossRef]
- Dhawi, F.; El-Beltagi, H.S.; Aly, E.; Hamed, A.M. Antioxidant, antibacterial activities and mineral content of buffalo yoghurt fortified with fenugreek and Moringa oleifera seed flours. Foods 2020, 9, 1157. [Google Scholar] [CrossRef]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT 2015, 63, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Bchir, B.; Bouaziz, M.A.; Blecker, C.; Attia, H. Physico-Chemical, antioxidant activities, textural, and sensory properties of yoghurt fortified with different states and rates of pomegranate seeds (Punica granatum L.). J.Texture Stud. 2020, 51, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, W.Y.; Yeon, S.J.; Choi, S.H.; Lee, C.H. Effects of lotus (Nelumbo nucifera) leaf on quality and antioxidant activity of yogurt during refrigerated storage. Food Sci. Anim. Resour. 2019, 39, 792. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.M.; Alqah, H.A.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT 2021, 137, 110389. [Google Scholar] [CrossRef]
- Marand, M.A.; Amjadi, S.; Marand, M.A.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Nguyen, L.; Hwang, E.S. Quality characteristics and antioxidant activity of yogurt supplemented with aronia (Aronia melanocarpa) juice. Prev. Nutr. Food Sci. 2016, 21, 330. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Jattar-Santiago, K.Y.; Avila-Sosa, R.; Pérez-Xochipa, I.; Guerrero-Beltrán, J.A.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Antioxidant fortification of yogurt with red cactus pear peel and its mucilage. CyTAJ. Food 2019, 17, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Suharto, E.L.S.; Arief, I.I.; Taufik, E. Quality and antioxidant activity of yogurt supplemented with roselle during cold storage. J. Exot. Pet. Med. 2016, 39, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.Y.; Yeon, S.J.; Hong, G.E.; Kim, J.H.; Tsend-Ayush, C.; Lee, C.H. Antioxidant activity and quality characteristics of yogurt added green olive powder during storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 865. [Google Scholar]
- Georgakouli, K.; Mpesios, A.; Kouretas, D.; Petrotos, K.; Mitsagga, C.; Giavasis, I.; Jamurtas, A.Z. The effects of an olive fruit polyphenol-enriched yogurt on body composition, blood redox status, physiological and metabolic parameters and yogurt microflora. Nutrients 2016, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Antioxidant activity and inhibition of key enzymes linked to type-2 diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013, 17, 295–301. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Zeppa, G. Yogurt enrichment with grape pomace: Effect of grape cultivar on physicochemical, microbiological and sensory properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Jovanović, M.; Petrović, M.; Miočinović, J.; Zlatanović, S.; LaličićPetronijević, J.; Mitić-Ćulafić, D.; Gorjanović, S. Bioactivity and sensory properties of probiotic yogurt fortified with apple pomace flour. Foods 2020, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Ojha, P.; Karki, T.B. Phytochemicals and syneresis of osmo-dried mulberry incorporated yoghurt. Food Sci. Nutr. 2018, 6, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.B. Storage quality and antioxidant properties of yogurt fortified with polyphenol extract from nutmeg, black pepper, and white pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial action of yoghurt enriched withwatermelon seed milk on renal injured hyperuricemic rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, physciochemcial and sensory properties of probitoic yoghurt made from whole milk powder reconstituted in aqueous fennel extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Buyuktuncer, Z.; Fisunoğlu, M.; Guven, G.S.; Unal, S.; Besler, H.T. The cholesterol lowering efficacy of plant stanol ester yoghurt in a Turkish population: A double-blind, placebo-controlled trial. Lipids Health Dis. 2013, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Párraga-Martínez, I.; López-Torres-Hidalgo, J.D.; Del Campo, J.M.; Galdón-Blesa, M.P.; Precioso-Yáñez, J.C.; Rabanales-Sotos, J.; Lloret-Callejo, Á. Long-term effects of plant stanols on the lipid profile of patients with hypercholesterolemia. A randomized clinical trial. Rev. Esp. Cardiol. 2015, 68, 665–671. [Google Scholar] [CrossRef]
- Vásquez-Trespalacios, E.M.; Romero-Palacio, J. Efficacy of yogurt drink with added plant stanol esters (Benecol®, Colanta) in reducing total and LDL cholesterol in subjects with moderate hypercholesterolemia: A randomized placebo-controlled crossover trial NCT01461798. Lipids Health Dis. 2014, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doornbos, A.M.E.; Meynen, E.M.; Duchateau, G.S.M.J.E.; Van der Knaap, H.C.M.; Trautwein, E.A. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2006, 60, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Mazza, E.; Salvati, M.; Santariga, E.; Giampà, S.; Spagnuolo, R.; Montalcini, T. Effects of a Portfolio-Mediterranean Diet and a Mediterranean Diet with or without a Sterol-Enriched Yogurt in Individuals with Hypercholesterolemia. Endocrino. Metabol. 2020, 35, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Volpe, R.; Niittynen, L.; Korpela, R.; Sirtori, C.; Bucci, A.; Fraone, N.; Pazzucconi, F. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br. J. Nutr. 2001, 86, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penchalaraju, M.; Kuna, A.; Shailaja, P.S.S.; Kumar, K.V.; Devi, P.U.; Supraja, T.; Jones, P.J. Cholesterol-lowering efficacy of plant sterol-enriched flavored milk, yogurt, fruit bar, and soya milk in mild hypercholesterolemic Indian subjects. Clin. J. Nutr. Diet 2018, 1, 1–6. [Google Scholar]
- Hyun, Y.J.; Kim, O.Y.; Kang, J.B.; Lee, J.H.; Jang, Y.; Liponkoski, L.; Salo, P. Plant stanol esters in low-fat yogurt reduces total and low-density lipoprotein cholesterol and low-density lipoprotein oxidation in normocholesterolemic and mildly hypercholesterolemic subjects. Nutr. Res. 2005, 25, 743–753. [Google Scholar] [CrossRef]
- Noakes, M.; Clifton, P.M.; Doornbos, A.M.; Trautwein, E.A. Plant sterol ester–enriched milk and yoghurt effectively reduce serum cholesterol in modestly hypercholesterolemic subjects. Eur. J. Nutr. 2005, 44, 214–222. [Google Scholar] [CrossRef]
- Rudkowska, I.; AbuMweis, S.S.; Nicolle, C.; Jones, P.J. Cholesterol-lowering efficacy of plant sterols in low-fat yogurt consumed as a snack or with a meal. J. Am. Coll. Nutr. 2008, 27, 588–595. [Google Scholar] [CrossRef]



| Study Country | Year Reported | Sample Size | Prevalence (%) | Reference | |
|---|---|---|---|---|---|
| Men | Women | ||||
| Spain | 2008–2010 | 11,554 | 25.5 | 26.4 | [4] |
| Kazakhstan | 2012–2015 | 954 | 28.4 | 44.1 | [9] |
| India | 2014 | 6123 | 25.1 | 24.9 | [41] |
| Saudi Arabia | 2016 | 1019 | 56.7 | 43.3 | [43] |
| France | 2014–2016 | 2321 | 27.8 | 19.0 | [44] |
| Cyprus | 2004–2005 | 150 | 60 | 68 | [45] |
| Kingdom of Bahrain | 2012 | 166 | 3 | 12.5 | [46] |
| Thailand | 2004 | 39,290 | 14 | 17 | [47] |
| Mexico | 1987–1988 | 33,588 | 10.6 | 10.6 | [48] |
| Iran | 2016 | 21,293 | 23.8 | 29.8 | [49] |
| United States | 2015–2016 | ND | 28.5 | 8.9 | [50] |
| Japan | 2010 | 2417 | 21.5 | 31 | [51] |
| Bangladesh | 2006 | 2610 | 2.2 | 0.5 | [52] |
| Malaysia | 2015 | 19,935 | 43.5 | 52.2 | [53] |
| Kuwait | 1995 | 1076 | 16 | 15.7 | [54] |
| Nutrients (per 100 g) | Whole Milk Yogurt | Low-Fat Yogurt | Non-Fat Yogurt |
|---|---|---|---|
| Energy (kcal) | 61 | 63 | 56 |
| Water (g) | 87.89 | 85.06 | 85.22 |
| Protein (g) | 3.47 | 5.25 | 5.73 |
| Fat total (g) | 3.25 | 1.55 | 0.18 |
| Sugar total (g) | 4.66 | 7.04 | 7.68 |
| Saturated fatty acids (g) | 2.096 | 1 | 0.116 |
| Monounsaturated fatty acids (g) | 0.893 | 0.426 | 0.049 |
| Polyunsaturated fatty acids (g) | 0.092 | 0.044 | 0.005 |
| Cholesterol (mg) | 13 | 6 | 2 |
| Carbohydrate (g) | 4.66 | 7.04 | 7.68 |
| Sodium (mg) | 46 | 70 | 77 |
| Vitamin A, RAE (µg) | 27 | 14 | 2 |
| Thiamin (mg) | 0.029 | 0.044 | 0.048 |
| Riboflavin (mg) | 0.142 | 0.214 | 0.234 |
| Niacin (mg) | 0.08 | 0.114 | 0.124 |
| Folate (µg) | 7.0 | 11 | 12 |
| Vitamin B12 (µg) | 0.37 | 0.56 | 0.61 |
| Vitamin K (µg) | 0.2 | 0.2 | 0.2 |
| Magnesium (mg) | 12 | 17 | 19 |
| Calcium (mg) | 121 | 183 | 199 |
| Iron (mg) | 0.05 | 0.08 | 0.09 |
| Phosphorus (mg) | 95 | 114 | 157 |
| Potassium (mg) | 155 | 234 | 255 |
| Zinc (mg) | 0.59 | 0.89 | 0.97 |
| Copper (mg) | 0.009 | 0.013 | 0.015 |
| Study Country | Chemical Composition and Physical Nature | Plant Name | Part Used | Quality Effects | Health Effects | Reference |
|---|---|---|---|---|---|---|
| Australia | ND/Set type | Pineapple (Ananas comosus L. Merrill) | Peel and pomace powder | Increase in probiotic population by 0.3–1.4 log cycle | Remarkable antioxidant activity in case of DPPH, (IC50 = 0.37–0.19 mg/mL) and hydroxyl radicals (58.52–73.55%) | [89] |
| Brazil | Full-fat milk/Set type | Strawberry (Fragaria × ananassa) | Juice | Higher total lactic acid bacteria count, i.e., 108 CFU/mL; Decrease in viscosity | Three-fold increase in total anthocyanins content; Antioxidant activity in DPPH was 8.86–9.19 mgGAE/mL and in ABTS it was 0.26–0.38 mgGAE/mL | [90] |
| Egypt | Full-fat milk/Set type | Green tea (Camellia sinensis L.) and Moringa oleifera | Leaves extract | No suppression of starter culture growth; No significant change in viscosity; green tea extract improved consistency | High total phenolic content in case of green tea extract (712 mgGAE/100 g) and Moringa (280 mgGAE/100 g); DPPH radical scavenging activity in case of green tea extract (LC50 = 64 µg/mL) and in Moringa (LC50 = 65 µg/mL) | [91] |
| Saudi Arabia | Full-fat milk/Set type | Fenugreek (Trigonella foenum-graecum) and Moringa oleifera | Seed extract | Increase in the viable count of Streptococcus thermophiles and Lactobacillus delbrueckii subsp. bulgaricus | Increase in total phenolic content and antioxidant activity; Increase in mineral content viz. Ca, P, K, Mg, Zn, and Fe; Antibacterial activity against Escherichia coli, Staphylococcus aureus, Lesteriamonocytogenes, and Salmonella spp. | [92] |
| Italy | Full-fat milk/Stirred | Hazelnut (Corylus avellana L.) | Skin | Increase the viable count of Streptococcus thermophiles (8.38 log10CFU/mL) and Lactobacillus delbrueckii subsp. bulgaricus (7.73 Log10CFU/mL) | Increase in total phenolic content (13.12–19.43 µgGAE/g) and an increase in antioxidant activity in DPPH (25.27–47.29 TEµM/g) | [93] |
| Egypt | Full-fat milk/Stirred | Cinnamon (Cinnamomum cassia) | Bark powder | Nd | Increase in total phenolic content (28.3 mg catechin/100 g); Fortified yogurt exhibited significantly higher radical scavenging activity than the plain yogurt both in the ABTS and DPPH assay ( p < 0.05) | [94] |
| Tunisia | Low-fat milk/Set type | Pomegranate (Punica granatum L.) | Seeds | Change in color; Decrease in firmness | Increase in antioxidant activity; Increase in acceptance (based on sensory) | [95] |
| South Korea | Low-fat milk/Stirred | Lotus (Nelumbo nucifera) | Leaf powder | No significant change in the viability of lactic acid bacteria; 4-fold increase in viscosity | Increase in total phenolic content (47.94–61.94 µgGAE/g) and DPPH activity (48.81–52.34%) | [96] |
| Saudi Arabia | Low-fat milk/Set type | Argel (Solenostemma argel Hayne) | Leaf extract | Increase in acidity, lactic acid bacteria count, water holding capacity, viscosity, and stability | High total phenolic content (23.79 mgGAE/100 g) and DPPH activity (36.39%) | [97] |
| Iran | Full-fat milk/Stirred | Flaxseed (Linumusitatissimum L.) | Powder | Increase in acidity, water holding capacity, and viscosity | Increase in DPPH scavenging capacity (45.83%) | [98] |
| South Korea | Low-fat milk/ND | Aronia (Aronia melanocarpa) | Juice | Increase in lactic acid bacteria count (9.59 logCFU/mL); Increase in total acidity | Increase in DPPH scavenging capacity (77.87%), ABTS (70.90%) and reducing power (29.86%); Increase in total phenolic content (54.05 mgGAE/g) and total flavonoids (152.10 mgCatechin/g) | [99] |
| Mexico | ND | Red cactus pear (Opuntia ficus-indica L.) | Peel and mucilage powder | Magenta color produced | Increase in total phenolic compounds, total flavonoids, total betalains, inhibition capacity, and reducing power, respectively | [100] |
| Indonesia | Full fat milk/ND | Roselle (Hibiscus sabdariffa L.) | Flower extract | Increase in viscosity, holding capacity; Decrease in total lactic acid bacteria count | Increase in DPPH scavenging capacity | [101] |
| South Korea | Low-fat milk/ND | Olive (Olea europaea) | Powder | Decrease in viscosity and total lactic acid bacteria count | At day zero storage, the total phenolic content (6.96 mgGAE/100 g), DPPH scavenging capacity (47.53%), and reducing power (0.57%) | [102] |
| Greece | Low-fat milk/ND | Olive (Olea europaea) | Polyphenols | Increase in lactic acid bacteria count | Decreased levels of low-density lipoprotein (LDL) cholesterol and thiobarbituric acid reactive substances | [103] |
| South Korea | Non-fat milk/ Set type | Green tea(Camellia sinensis L.) | Powder | Increase in lactic acid bacteria count | Decreased expression of TNF-α and IL-1β in a human colorectal cell line, HT-29 | [104] |
| Malaysia | Full-fat milk/ND | Neem (Azadirachta indica) | Leaves powder | Nd | Increase in total phenolic content (74.9–19 µgGAE/mL) and increase in antioxidant activity in DPPH (53.1%); Increase in enzymes inhibition (α-amylase 47.4%, α-glucosidase 15.2% and angiotensin-1 converting enzyme 48.4) | [105] |
| Italy | Full-fat milk/ND | Grape (Vitis vinifera) | Skin powder | Decrease in syneresis and fat; Increase in acidity | Increase in total phenolic content, and antioxidant activity | [106] |
| Serbia | Full-fat milk/Set type | Apple (Malus domestica) | Pomace flour | Increase the firmness and viscosity | Increase in total phenolic content, radical scavenging (DPPH), and reducing activity (FRAP); Inhibit colon cancer cells’ viability | [107] |
| Nepal | Full-fat milk/Set type | Mulberry (Morus L.) | Osmo- dried fruit | Reduce in syneresis | Increase in total phenolic content (68.03 mgGAE/100 g), an increase in anthocyanins content (7.9 mg/100 g), and an increase in antioxidant activity in DPPH (47.6%) | [108] |
| Saudi Arabia | Full-fat milk/ND | Nutmeg (Myristica fragrans Houtt.), Black pepper and white pepper (Piper nigrum L.) | Water extract | High production of lactic acid | Radical scavenging activity was positively affected; High total phenolic content | [109] |
| Egypt | Full- fat milk/ND | Watermelon (Citrullus lanatus) | Seed milk | Apperance, flavor, body and texture, and overall acceptability was best with 50% cow’s milk and 50% watermelon seed milk | Improved the renal function in hyperuricemic rats; Enhancment of the activities of superoxide dismutase, catalase, and glutathione transferase | [110] |
| Egypt | Whole milk powder/ | Fennel (Foeniculum vulgare) | Seed water extract | Titratable acidity significantly decreased | Antioxidant activity significantly increased; Total phenolic content increased | [111] |
| Study County | Type of Study | Sample Size (n)/ Mean Age (Years) | Characteristics of the Subject | Dosage Concentration | Period of Intervention | Effect | References |
|---|---|---|---|---|---|---|---|
| Italy | Retrospective | 24/52 ± 12 | BMI (kg/m2): 27.3 ± 2; hypercholesterolemia | Sterol enriched yogurt (1.6 g/day) | 48 days | ↓ LDL (23 ± 4 mg/dL) | [116] |
| Turkey | Randomized | 35/45.5 | BMI (kg/m2): 27.9 ± 3.15; untreated mild to moderate hypercholesterolemia | Sterol enriched (1/9 g/day) low-fat yogurt(115 g/day) | 4 weeks | ↓Serum total cholesterol (4.6%) ↓LDL (6.3%) | [112] |
| Spain | Randomized | 91/54.8 | BMI (kg/m2): 28.3; hypercholesterolemia | Stanol enriched (2 g/day) yogurt | 12 months | ↓ LDL (13.7 mg/dL) | [113] |
| Colombia | Randomized | 40/37.9 | BMI (kg/m2): 25.0; moderate hypercholesterolemia | Stanol enriched yogurt 4 g (2 pots/day) | 4 weeks | ↓ Serum total cholesterol (7.2%) ↓LDL (10.3%) | [114] |
| Italy | Randomized | 30/ND | BMI (kg/m2): 24.6; moderate hypercholesterolemia | Sterol enriched (1–2 g/day) low-fat low-lactose yogurt | 8 weeks | ↓ LDL | [117] |
| India | ND | 48/ND | BMI (kg/m2): ND; mild hypercholesterolemia | Sterol enriched yogurt (200 g/day) | 30 days | ↓ Serum total cholesterol (4.3%) ↓LDL (5.3%) | [118] |
| South Korea | Randomized | 51/28.5 | BMI (kg/m2): 22.8; mild hypercholesterolemia | Stenol enriched (2 g/day) 150 mL strawberry yogurt | 4 weeks | ↓ Serum total cholesterol (6%) ↓LDL (10%) | [119] |
| Australia | Randomized | 42/60.4 | BMI (kg/m2): 26.5; moderate hypercholesterolemia | Sterol enriched (1.8 g/day) and stanol enriched (1.7 g/day) low-fat yogurt (300 g/day) | 3 weeks | ↓ LDL (6%) ↓ LDL (5%) | [120] |
| Netherlands | Randomized | 184/57 | BMI (kg/m2): 25.2; moderate hypercholesterolemia | Sterol enriched (3 g/day) yogurt (100 g/day) | 4 weeks | ↓ LDL (9.3–9.5%) | [115] |
| Canada | Randomized | 26/59.6 | BMI (kg/m2): 26.4; hypercholesterolemia | Sterol enriched (1.6 g/day) low-fat yogurt | 4 weeks | ↓ LDL (8.69%) | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Bhardwaj, K.; Cruz-Martins, N.; Sharma, R.; Siddiqui, S.A.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Dantas, A.; Verma, R.; et al. Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach. Molecules 2022, 27, 3479. https://doi.org/10.3390/molecules27113479
Kumar H, Bhardwaj K, Cruz-Martins N, Sharma R, Siddiqui SA, Dhanjal DS, Singh R, Chopra C, Dantas A, Verma R, et al. Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach. Molecules. 2022; 27(11):3479. https://doi.org/10.3390/molecules27113479
Chicago/Turabian StyleKumar, Harsh, Kanchan Bhardwaj, Natália Cruz-Martins, Ruchi Sharma, Shahida Anusha Siddiqui, Daljeet Singh Dhanjal, Reena Singh, Chirag Chopra, Adriana Dantas, Rachna Verma, and et al. 2022. "Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach" Molecules 27, no. 11: 3479. https://doi.org/10.3390/molecules27113479
APA StyleKumar, H., Bhardwaj, K., Cruz-Martins, N., Sharma, R., Siddiqui, S. A., Dhanjal, D. S., Singh, R., Chopra, C., Dantas, A., Verma, R., Dosoky, N. S., & Kumar, D. (2022). Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach. Molecules, 27(11), 3479. https://doi.org/10.3390/molecules27113479