nor 3′-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis
Abstract
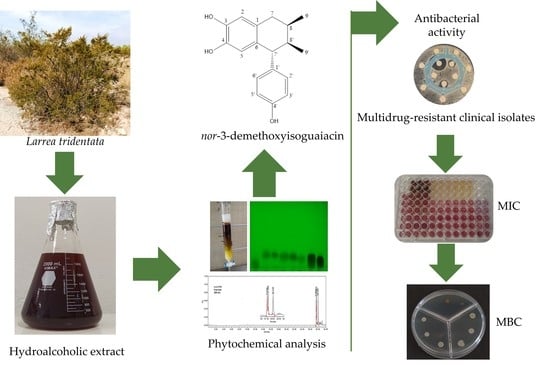
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial Sensitivity Test of Multidrug-Resistant Clinical Isolates
2.2. Antibacterial Activity
2.2.1. Chemical Characterization of the Extract
2.2.2. Identification of Major Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Hydroalcoholic Extract
3.3. Chemical Characterization of the Extract
3.4. Identification of Major Compounds
3.5. Bacterial Strains and Culture Conditions
3.6. Antimicrobial Sensitivity Test of Field Strains
3.7. Antibacterial Activity
3.7.1. Minimal Inhibitory Concentration
3.7.2. Minimal Bactericidal Concentration
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Motaung, T.E.; Petrovski, K.R.; Petzer, I.M.; Thekisoe, O.; Tsilo, T.J. Importance of bovine mastitis in Africa. Anim. Health Res. Rev. 2017, 18, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal-Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procópio, T.; Moura, M.C.; Bento, E.F.; Soares, T.; Coelho, L.C.; Bezerra, R.; Mota, R.; Porto, A.; Paiva, P.; Napoleão, T. Looking for alternative treatments for bovine and caprine mastitis: Evaluation of the potential of Calliandra surinamensis leaf pinnulae lectin (CasuL), both alone and in combination with antibiotics. Microbiologyopen 2019, 8, e869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments-A review. Asian-Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Sadaf, N.; Ahmed, K.; Nafees, M.; Noor, T.K. Prevalence of sub-clinical mastitis, identification of causative agents and sensitivity profile of isolates in Northern Pakistan. Nat. Sci. 2016, 14, 7–10. [Google Scholar] [CrossRef]
- Papić, B.; Golob, M.; Kušar, D.; Pate, M.; Zdovc, I. Source tracking on a dairy farm reveals a high occurrence of subclinical mastitis due to hypervirulent Listeria monocytogenes clonal complexes. J. Appl. Microbiol. 2019, 127, 1349–1361. [Google Scholar] [CrossRef]
- Jamali, H.; Radmehr, B. Frequency, virulence genes and antimicrobial resistance of Listeria spp. isolated from bovine clinical mastitis. Vet. J. 2013, 198, 541–542. [Google Scholar] [CrossRef]
- Olde-Riekerink, R.G.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.Y.; Leblond, A.; Haenni, M.; Gay, É. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Ruegg, P.L. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomazi, T.; Dos Santos, M.V. Antimicrobial use for treatment of clinical mastitis in dairy herds from Brazil and its association with herd-level descriptors. Prev. Vet. Med. 2020, 176, 104937. [Google Scholar] [CrossRef]
- Ferri, M.; Ran|ucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Warda, A.K.; Hill, C.; Ross, R.P. Non-antibiotic microbial solutions for bovine mastitis—Live biotherapeutics, bacteriophage, and phage lysins. Crit. Rev. Microbiol. 2019, 45, 564–580. [Google Scholar] [CrossRef]
- Gomes, F.; Rodrigues, M.E.; Martins, N.; Ferreira, I.C.F.R.; Henriques, M. Phenolic Plant Extracts Versus Penicillin G: In Vitro Susceptibility of Staphylococcus aureus Isolated from Bovine Mastitis. Pharmaceuticals 2019, 31, 128. [Google Scholar] [CrossRef] [Green Version]
- Okmen, G.; Balpınar, N. The biological activities of Hypericum perforatum L. Afr. J. Tradit. Complement. Altern. Med. 2016, 23, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, A.P.; Estrela, F.T.; Moraes, T.S.; Carneiro, L.J.; Bastos, J.K.; dos Santos, R.A.; Ambrósio, S.R.; Martins, C.H.; Veneziani, R.C. In vitro antimicrobial activity of plant-derived diterpenes against bovine mastitis bacteria. Molecules 2013, 18, 7865–7872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldívar, R.H.L. Estado actual del conocimiento sobre las propiedades biocidas de la gobernadora [Larrea tridentata (DC)Coville]. Rev. Mex. Fitopatol. 2003, 21, 214–222. [Google Scholar]
- Arteaga, S.; Andrade-Cetto, A.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Skouta, R.; Morán-Santibañez, K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef] [Green Version]
- Siddique, Y.H.; Ali, F. Protective effect of nordihydroguaiaretic acid (NDGA) on the transgenic Drosophila model of Alzheimer’s disease. Chem. Biol. Interact. 2017, 269, 59–66. [Google Scholar] [CrossRef]
- Bashyal, B.; Li, L.; Bains, T.; Debnath, A.; LaBarbera, D.V. Larrea tridentata: A novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PLoS Negl. Trop. Dis. 2017, 11, e0005832. [Google Scholar] [CrossRef] [Green Version]
- Delgadillo-Ruíz, L.; Bañuelos-Valenzuela, R.; Delgadillo-Ruíz, O.; Silva-Vega, M.; Gallegos-Flores, P. Composición química y efecto antibacteriano in vitro de extractos de Larrea tridentata, Origanum vulgare, Artemisa ludoviciana y Ruta graveolens. Nova Sci. 2017, 9, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Bañuelos-Valenzuela, R.; Delgadillo-Ruiz, L.; Echavarría-Cháirez, F.; Delgadillo-Ruiz, O.; Meza-López, C. Composición química y FTIR de extractos etanólicos de Larrea tridentata, Origanum vulgare, Artemisa ludoviciana y Ruta graveolens. Agrociencia 2018, 52, 309–321. [Google Scholar]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Avila-Ramos, F.; Aquino-Torres, E.; Prieto-Méndez, J.; Hetta, H.F.; El-Saber, B.G.; Z aragoza-Bastida, A. Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria. Agronomy 2021, 11, 957. [Google Scholar] [CrossRef]
- Gerstel, J.; Turner, T.; Ruiz, G.; Wise, J.; Stein, A.; Jones, G.; Morin, T.; Pinazza, T.; Sukhorukov, E.; Clark, D.; et al. Identification of botanicals with potential therapeutic use against methicillin-resistant Staphylococcus aureus (MRSA) infections. Phytother. Res. 2018, 32, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Melo, K.; García, A.; Romo-Mancillas, A.; Garza-González, E.; Rivas-Galindo, V.M.; Miranda, L.D.; Vargas-Villarreal, J.; Favela-Hernández, J.M.J.; Camacho-Corona, M. del. R. meso-Dihydroguaiaretic acid derivatives with antibacterial and antimycobacterial activity. Bioorg. Med. Chem. 2017, 25, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Amorim, E.L.C.; Sobrinho, T.J.S.P.; Saraiva, A.M.; Pisciottano, M.N.C.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Antibacterial activity of crude methanolic extract and fractions obtained from Larrea tridentata leaves. Ind. Crops Prod. 2013, 41, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.; Rodríguez, R.; Ruiz, J.; Morales-Adame, D.; Castillo, F.; Hernández-Castillo, F.D.; Aguilar, C.N. Antibacterial activity of plant extracts obtained with alternative organics solvents against food-borne pathogen bacteria. Ind. Crops Prod. 2012, 37, 445–450. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; Camacho-Corona, M. del R. Potential mechanism of action of 3’-demethoxy-6-O-demethyl-isoguaiacin on methicillin resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef]
- Turner, T.; Ruiz, G.; Gerstel, J.; Langland, J. Characterization of the antibacterial activity from ethanolic extracts of the botanical, Larrea tridentata. BMC Complement. Med. Ther. 2021, 21, 177. [Google Scholar] [CrossRef]
- Cunningham-Oakes, E.; Soren, O.; Moussa, C.; Rathor, G.; Liu, Y.; Coates, A.; Hu, Y. Nordihydroguaiaretic acid enhances the activities of aminoglycosides against methicillin-sensitive and resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2015, 6, 1195. [Google Scholar] [CrossRef] [Green Version]
- Clemente-Soto, A.F.; Balderas-Rentería, I.; Rivera, G.; Segura-Cabrera, A.; Garza-González, E. del. R. Camacho-Corona, M. Potential mechanism of action of meso-dihydroguaiaretic acid on Mycobacterium tuberculosis H37Rv. Molecules 2014, 19, 20170–20182. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Beltrán, S.; Rubio-Badillo, M.Á.; Juárez, E.; Hernández-Sánchez, F.; Torres, M. Nordihydroguaiaretic acid (NDGA) and α-mangostin inhibit the growth of Mycobacterium tuberculosis by inducing autophagy. Int. Immunopharmacol. 2016, 31, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalik, M.; Podbielska-Kubera, A.; Samet, A.; Konopka, W. Multidrug-resistant strains of coagulase-negative staphylococci isolated from patients with chronic sinusitis—MDR, XDR, PDR strains. Otolaryngol. Pol. 2019, 74, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gregova, G.; Kmet, V. Antibiotic resistance and virulence of Escherichia coli strains isolated from animal rendering plant. Sci. Rep. 2020, 10, 17108. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically (Approved Standards); CLSI document M7-A5; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Bocanegra-García, V.; Del Rayo Camacho-Corona, M.; Ramírez-Cabrera, M.; Rivera, G.; Garza-González, E. The bioactivity of plant extracts against representative bacterial pathogens of the lower respiratory tract. BMC Res. Notes 2009, 2, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itzá-Ortiz, M.; Chávez, J.; Urquizo, E.; Suescún, J. Phytobiotic activity of Larrea tridentata, Origanum vulgare and Plectranthus amboinicus in gram positive and gram negative bacterias. Interciencia 2019, 44, 298–302. [Google Scholar]
- Ruiz, J.; Ascacio-Valdés, J.; Rodriguez, R.; Morales, D.; Aguilar, C. Phytochemical screening of extracts from some Mexican plants used in traditional medicine. J. Med. Plants 2011, 5, 2791–2797. [Google Scholar]
- Martins, S.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Sep. Purif. Technol. 2012, 88, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Salud-Pérez, G.; Cuauhtemoc-Pérez, G.; Zavala, S.M.A. A study of the antidiarrheal properties of Loeselia mexicana on mice and rats. Phytomedicine 2005, 12, 670–674. [Google Scholar] [CrossRef]
- Aldana, J.; Téllez, N.; Gamboa, F. Antimicrobial activity of fractions and subfractions of Elaeagia utilis against microorganisms of importance in dental caries. Acta Odontol. Latinoam. 2013, 26, 104–111. [Google Scholar]
- Rodríguez-Garza, R.G.; González-González, G.; Verde-Star, M.; Morales-Rubio, M.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Nuñez- González, N.A.; Treviño-Neavez, J.F. Bioprospección de la actividad antimicótica de extractos metanólicos de Ariocarpus kotschoubeyanus y Ariocarpus retusus. Polibotánica 2011, 31, 143–155. [Google Scholar]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernández- Hernández, S.; Wei, X.; Fan, M. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef] [PubMed]
- Mazhangara, I.R.; Idamokoro, E.M.; Chivandi, E.; Afolayan, A.J. Phytochemical screening and in vitro evaluation of antioxidant and antibacterial activities of Teucrium trifidum crude extracts. Heliyon 2020, 6, e04395. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Xiao, X.N.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.Z.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.M.; Delle Monache, F.; Smânia, A., Jr. Antibacterial activity of coumarins. Z. Naturforsch. C J. Bio. Sci. 2005, 60, 693–700. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-Sánchez, N.G.; Gómez-Rivera, A.; Alvarez-Fitz, P.; Ventura-Zapata, E.; Pérez-García, M.D.; Avilés-Flores, M.; Gutiérrez-Román, A.S.; González-Cortazar, M. Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog. 2019, 128, 347–353. [Google Scholar] [CrossRef]
- Wang, H.; Zou, D.; Xie, K.; Xie, M. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014, 10, 2341–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L.; Poroikov, V.; Petrou, A.; Geronikakim, A.; Ciricm, A.; Glamoclijam, J.; Sokovicm, M. New Caffeic Acid Derivatives as Antimicrobial Agents: Design, Synthesis, Evaluation and Docking. Curr. Top. Med. Chem. 2019, 19, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.; Lowen, N.; Blake, D.J. Caffeic Acid Esters Are Effective Bactericidal Compounds Against Paenibacillus larvae by Altering Intracellular Oxidant and Antioxidant Levels. Biomolecules 2019, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.N.; Shi, X.W.; Kang, L.Y.; Niu, L.Y.; Wang, X.G.; Feng, W. Clerodens E-J, antibacterial caffeic acid derivatives from the aerial part of Clerodendranthus spicatus. Fitoterapia 2016, 114, 110–114. [Google Scholar] [CrossRef]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical Study and Antibacterial and Antibiotic Modulation Activity of Punica granatum (Pomegranate) Leaves. Scientifica 2020, 2020, 8271203. [Google Scholar] [CrossRef] [Green Version]
- Motallebi, M.; Khorsandi, K.; Sepahy, A.A.; Chamani, E.; Hosseinzadeh, R. Effect of rutin as flavonoid compound on photodynamic inactivation against P. aeruginosa and S. aureus. Photodiagnosis Photodyn. Ther. 2020, 32, 102074. [Google Scholar] [CrossRef]
- Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Vázquez-Sánchez, M.; Álvarez-Bernal, D.; Oregel-Zamudio, E.; Ceja-Torres, L.F.; Medina-Medrano, J.R. Quantitative Analysis of Rutin by HPTLC and In Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina sphaerocephala. Plants 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Benabderrahmane, W.; Lores, M.; Benaissa, O.; Lamas, J.P.; de Miguel, T.; Amrani, A.; Benayache, F.; Benayache, S. Polyphenolic content and bioactivities of Crataegus oxyacantha L. (Rosaceae). Nat. Prod. Res. 2021, 35, 627–632. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.F.; de Oliveira, R.G.; Mendes Soares, I.; da Costa Alvim, T.; Donizeti Ascêncio, S.; de Oliveira Martins, D.T. Evaluation of acute toxicity, antibacterial activity, and mode of action of the hydroethanolic extract of Piper umbellatum L. J. Ethnopharmacol. 2014, 151, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananth, D.A.; Rameshkumar, A.; Jeyadevi, R.; Jagadeeswari, S.; Nagarajan, N.; Renganathan, R.; Sivasudha, T. Antibacterial potential of rutin conjugated with thioglycolic acid capped cadmium telluride quantum dots (TGA-CdTe QDs). Spectrochim. Acta A 2015, 138, 684–692. [Google Scholar] [CrossRef]
- Mambe, F.T.; Na-Iya, J.; Fotso, G.W.; Ashu, F.; Ngameni, B.; Ngadjui, B.T.; Beng, V.P.; Kuete, V. Antibacterial and Antibiotic Modifying Potential of Crude Extracts, Fractions, and Compounds from Acacia polyacantha Willd. against MDR Gram-Negative Bacteria. Evid Based Complement. Altern. Med. 2019, 2019, 7507549. [Google Scholar] [CrossRef] [Green Version]
- Cartaya, O.; Reynaldo, I. Flavonoides: Características químicas y aplicaciones. Cult. Trop 2001, 22, 5–14. [Google Scholar]
- Martínez-Aguilar, Y.; Soto-Rodríguez, F.; Almeida-Saavedra, M.; Hermosilla-Espinosa, R.; Martínez-Yero, O. Metabolitos secundarios y actividad antibacteriana in vitro de extractos de hojas de Anacardium occidentale L. (marañón). Rev. Cubana. Plan. Med. 2012, 17, 320–329. [Google Scholar]
- Gómez-Guiñán, Y.; Hidalgo, J.; Jiménez, M.; Salcedo, J. Obtención de extractos orgánicos con actividad antimicrobiana a partir de Penicillium sp. (Moniliales) aislado de la esponja Ircinia felix (Porifera: Demospongiae). Rev. Biol. Trop 2003, 51, 141–147. [Google Scholar]
- Olmedo-Juárez, A.; Briones-Robles, T.I.; Zaragoza-Bastida, A.; Zamilpa, A.; Ojeda-Ramírez, D.; Mendoza de Gives, P.; Olivares-Pérez, J.; Rivero-Perez, N. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) Willd against important bacteria in public health. Microb. Pathog. 2019, 136, 103660. [Google Scholar] [CrossRef]
- Dietz, B.M.; Chen, S.N.; Alvarenga, R.F.R.; Dong, H.; Nikolić, D.; Biendl, M.; van Breemen, R.B.; Bolton, J.L.; Pauli, G.F. DESIGNER Extracts as Tools to Balance Estrogenic and Chemopreventive Activities of Botanicals for Women’s Health. J. Nat. Prod. 2017, 80, 2284–2294. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Bowers, M.D. Chemical ecology of fruit defence: Synergistic and antagonistic interactions among amides from Piper. Funct. Ecol. 2014, 28, 1094–1106. [Google Scholar] [CrossRef]
- Fernández, S.; Hurtado, L.M.; Hernández, F. Fungicidal Components of Creosote Bush Resin. In Synthesis of Pesticides Chemical Structure and Biological Activity Natural Products with Biological Activity; Geissbühler, H., Ed.; Pergamon: Amsterdam, The Netherlands, 1979; pp. 351–355. [Google Scholar]
- Konno, C.; Lu, Z.Z.; Xue, H.Z.; Erdelmeier, C.A.; Meksuriyen, D.; Che, C.T.; Cordell, G.A.; Soejarto, D.D.; Waller, D.P.; Fong, H.H. Furanoid lignans from Larrea tridentata. J. Nat. Prod. 1990, 53, 396–506. [Google Scholar] [CrossRef] [PubMed]
- Snowden, R.; Harrington, H.; Morrill, K.; Jeane, L.; Garrity, J.; Orian, M.; Lopez, E.; Rezaie, S.; Hassberger, K.; Familoni, D.; et al. Comparison of the anti-Staphylococcus aureus activity of extracts from commonly used medicinal plants. J. Altern. Complement. Med. 2014, 20, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical Constituents of Salix babylonica L. and Their Antibacterial Activity Against Gram-Positive and Gram-Negative Animal Bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soberón, J.R.; Sgariglia, M.A.; Dip Maderuelo, M.R.; Andina, M.L.; Sampietro, D.A.; Vattuone, M.A. Antibacterial activities of Ligaria cuneifolia and Jodina rhombifolia leaf extracts against phytopathogenic and clinical bacteria. J. BioSci. Bioeng. 2014, 118, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Cáceres, R.R.; Luis Stephano-Hornedo, J.; Lugo, J.; Vaca, R.; Del Aguila, P.; Yañez-Ocampo, G.; Mora-Herrera, M.E.; Camacho-Díaz, L.M.; Cipriano-Salazar, M.; Alaba, P.A. Bactericidal effect of silver nanoparticles against propagation of Clavibacter michiganensis infection in Lycopersicon esculentum Mill. Microb. Pathog. 2018, 115, 358–362. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 25 October 2021).
- Rivero-Perez, N.; Hernández-Alvarado, J.L.; Valladares-Carranza, B.; Delgadillo-Ruiz, L.; Ojeda-Ramírez, D.; Sosa-Gutiérrez, C.G.; Morales-Ubaldo, A.L.; Vega-Sánchez, V.; Zaragoza-Bastida, A. Salix babylonica L. as a Natural Anticoccidial Alternative in Growing Rabbits. Evid Based Complement. Alternat. Med. 2019, 2019, 2107231. [Google Scholar] [CrossRef] [Green Version]
- Rangel-López, L.; Rivero-Perez, N.; Valladares-Carranza, B.; Olmedo-Juárez, A.; Delgadillo-Ruiz, L.; Vega-Sánchez, V.; Hori-Oshima, S.; Nassan, M.A.; Batiha, G.E.; Zaragoza-Bastida, A. Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance. Animals 2022, 12, 511. [Google Scholar] [CrossRef]
- Zaragoza-Bastida, A.; Flores-Aguilar, S.C.; Aguilar-Castro, L.M.; Morales-Ubaldo, A.L.; Valladares-Carranza, B.; Rangel-López, L.; Olmedo-Juarez, A.; Rosenfeld-Miranda, C.E.; Rivero-Perez, N. Antibacterial and Hemolytic Activity of Crotalus Triseriatus and Crotalus Ravus Venom. Animals 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Rangel-López, L.; Zaragoza-Bastida, A.; Valladares-Carranza, B.; Peláez-Acero, A.; Sosa-Gutiérrez, C.G.; Hetta, H.F.; Batiha, G.E.; Alqahtani, A.; Rivero-Perez, N. In Vitro Antibacterial Potential of Salix babylonica Extract against Bacteria that Affect Oncorhynchus mykiss and Oreochromis spp. Animals 2020, 10, 1340. [Google Scholar] [CrossRef]



| Antimicrobial | S. aureus01 | S. aureus02 | B. cereus | E. coli01 | E. coli02 | K. pneumoniae | P. multocida |
|---|---|---|---|---|---|---|---|
| Amikacin | 15 (I) | 7 (R) | 20 (S) | 12 (R) | 7 (R) | 12 (R) | 21 (S) |
| Ampicillin | 7 (R) | 7 (R) | 7 (R) | 11 (R) | 7 (R) | 7 (R) | 7 (R) |
| Carbenicillin | 22 (R) | 15(R) | 7 (R) | 11 (R) | 7 (R) | 7 (R) | 25 (S) |
| Cephalothin | 26 (R) | 35 (S) | 11 (R) | 12 (R) | 7 (R) | 7 (R) | 7 (R) |
| Cefotaxime | 14 (R) | 16 (I) | 8 (R) | 25 (I) | 26 (S) | 25 (I) | 26 (S) |
| Ciprofloxacin | 23 (S) | 11(R) | 20 (I) | 22 (S) | 25 (S) | 30 (S) | 29 (S) |
| Clindamycin | 30 (S) | 23 (S) | 30 (S) | 14 (R) | 7 (R) | 7 (R) | 7 (R) |
| Chloramphenicol | 25 (S) | 20 (R) | 20 (S) | 7 (R) | 7 (R) | 21 (S) | 27 (S) |
| Dicloxacillin | 7 (R) | 7 (R) | 7 (R) | 7 (R) | 7 (R) | 7 (R) | 7 (R) |
| Erythromycin | 16 (I) | 24 (S) | 25 (S) | 7 (R) | 7 (R) | 7 (R) | 18 (R) |
| Gentamicin | 15 (S) | 12 (R) | 22 (S) | 18 (S) | 18 (S) | 20 (S) | 15 (S) |
| Netilmicin | 11 (R) | 7 (R) | 15 (S) | 10 (R) | 17(S) | 10 (R) | 12 (R) |
| Nitrofurantoin | 22 (S) | 7 (R) | 16 (I) | 15 (I) | 7 (R) | 25 (S) | 28 (S) |
| Norfloxacin | 16 (I) | 7 (R) | 18 (S) | 20 (S) | 7 (R) | 15 (I) | 20 (S) |
| Penicillin | 30 (S) | 7 (R) | 7 (R) | 7 (R) | 7 (R) | 7 (R) | 7 (R) |
| Sulfamethoxazole/ Trimethoprim | 7 (R) | 7 (R) | 18 (S) | 7 (R) | 27 (S) | 16 (S) | 20 (S) |
| Tetracycline | 22 (S) | 16 (I) | 17 (I) | 7 (R) | 15 (S) | 7 (R) | 20 (S) |
| Vancomycin | 11 (R) | 7 (R) | 17 (S) | 7 (R) | 7 (R) | 7 (R) | 7 (R) |
| Reference Strains | MDR Clinical Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluated Treatment | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 |
| LTHE | 0.39 B,c | 3.12 D,d | 12.50 F,c | 12.50 F,c | 0.39 B,b | 0.19 A,e | 0.78 C,g | 6.25 E,c | 25.00 G,e | 6.25 E,c | 0.78 C,d |
| LTAq-F | 1.56 A,d | 1.56 A,c | 3.12 B,b | 3.12 B,b | SA | SA | SA | 12.5 D,d | 25.00 E,e | 6.25 C,c | SA |
| LTEtOAc-F | 0.04 A,a | 0.04 A,a | 3.12 D,b | 3.12 D,b | 0.39 C,b | 0.19 B,e | 0.19 B,e | 3.12 D,b | 3.12 D,c | 3.12 D,b | 0.39 C,c |
| Ltc1-F3 | 0.09 B,b | 0.09 B,b | 1.56 E,a | 3.12 F,b | 0.02 A,a | 0.02 A,b | 0.02 A,b | 0.78 D,a | 0.78 D,b | 0.39 C,a | 0.09 B,a |
| Ltc1-F4 | 0.09 C,b | 0.09 C,b | 3.12 G,b | 3.12 G,b | 0.02 A,a | 0.04 B,c | 0.04 B,c | 0.78 F,a | 0.78 F,b | 0.39 E,a | 0.19 D,b |
| Ltc1-F5 | 0.04 B,a | 0.09 B,b | 3.12 E,b | 3.12 E,b | 0.02 A,a | 0.09 B,d | 0.09 B,d | 0.78 D,a | 0.78 D,b | 0.39 C,a | 0.09 B,a |
| Ltc1-F6 | 0.09 A,b | 0.09 A,b | 3.12 D,b | 3.12 D,b | 0.78 C,c | 0.39 B,f | 0.39 B,f | 3.12 D,b | 6.25 E,d | 3.12 D,b | 0.78 C,d |
| C1 | NA | 0.09 C,b | 1.56 F,a | 3.12 G,b | 0.02 B,a | 0.01 A,a | 0.01 A,a | 0.78 E,a | 0.39 D,a | 0.39 D,a | 0.09 C,a |
| C2 | NA | NA | NA | 1.56 A,a | NA | NA | NA | NA | NA | NA | NA |
| Kanamycin * | 2 | 2 | 2 | 64 | 4 | 0.5 | 0.25 | 2 | 4 | 4 | 0.5 |
| p-value | 0.0001 | ||||||||||
| Position | δ1H (δ in ppm, J in Hz) 1 | δ13C 1 | Reported δ13C 1 a |
|---|---|---|---|
| 1 | 128.5 | 128.1 | |
| 2 | 6.45 (s) | 116.0 | 115.9 |
| 3 | 144.1 | 144 | |
| 4 | 144.4 | 144.4 | |
| 5 | 6.16 (s) | 118.0 | 117.7 |
| 6 | 130.8 | 130.7 | |
| 7 a b | 2.75 (dd, 6.6, 16.1) 2.32 (dd, 5.1, 16.1) | 35.9 | 35.7 |
| 8 | 1.92 (m) | 30.5 | 30.1 |
| 9 | 0.82 (d, 5.1) | 16.1 | 16.1 |
| 1′ | 139.7 | 139.3 | |
| 2′ | 6.76 (d, 8.4) | 130.9 | 130.8 |
| 3′ | 6.67 (d, 8.4) | 115.6 | 115.7 |
| 4′ | 156.1 | 156.3 | |
| 5′ | 6.67 (d, 8.4) | 115.6 | 115.7 |
| 6′ | 6.76 (d, 8.4) | 130.9 | 130.8 |
| 7′ | 3.46 (d, 6.2) | 51.1 | 50.8 |
| 8′ | 1.81 (dd, 2.5, 6.6) | 42.2 | 41.8 |
| 9′ | 0.8 (d, 6.2) | 16.1 | 16.3 |
| Reference Strains | MDR Clinical Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluated Treatment | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 |
| LTHE | 0.78 B,c | 6.25 D,c | 25.00 F,d | 25.00 F,c | 1.56 C,d | 0.39 A,d | 1.56 C,e | 25.00 F,d | 50.00 G,d | 12.50 E,d | 1.56 C,d |
| LTAq-F | 3.12 A,d | 3.12 A,b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| LTEtOAc-F | 0.09 A,a | 0.09 A,a | 6.25 D,c | 6.25 D,b | 0.78 C,c | 0.39 B,d | 0.39 B,c | 6.25 D,c | 6.25 D,c | 6.25 D,c | 0.78 C,c |
| Ltc1-F3 | NA | 0.09 B,a | NA | 6.25 D,b | 0.09 B,b | 0.09 B,b | 0.04 A,a | 3.12 C,b | 3.12 C,b | 3.12 C,b | 0.19 C,a |
| Ltc1-F4 | NA | NA | 6.25 E,c | 3.12 D,a | 0.09 B,b | 0.09 B,b | 0.04 A,a | 3.12 D,b | 3.12 D,b | 3.12 D,b | 0.39 C,b |
| Ltc1-F5 | 0.19 B,b | NA | 3.12 D,b | 3.12 D,a | 0.09 A,b | 0.19 B,c | 0.09 A,b | 6.25 E,c | 6.25 E,c | 6.25 E,c | 0.78 C,c |
| Ltc1-F6 | 0.09 A,a | 0.09 A,a | 6.25 E,c | 3.12 D,a | 1.56 C,d | SA | 0.78 B,d | 6.25 E,c | 6.25 E,c | 6.25 E,c | 1.56 C,d |
| C1 | NA | 0.09 C,a | 1.56 F,a | 3.12 G,a | 0.04 B,a | 0.02 A,a | 0.78 E,d | 1.56 F,a | 1.56 F,a | 1.56 F,a | 0.19 D,a |
| C2 | NA | NA | NA | 3.12 A,a | NA | NA | NA | NA | NA | NA | NA |
| Kanamycin * | 4 | 4 | 0.5 | 128 | 4 | 8 | 0.5 | 4 | 8 | 8 | 1 |
| p-value | 0.0001 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ubaldo, A.L.; Gonzalez-Cortazar, M.; Zaragoza-Bastida, A.; Meza-Nieto, M.A.; Valladares-Carranza, B.; A. Alsayegh, A.; El-Saber Batiha, G.; Rivero-Perez, N. nor 3′-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis. Molecules 2022, 27, 3620. https://doi.org/10.3390/molecules27113620
Morales-Ubaldo AL, Gonzalez-Cortazar M, Zaragoza-Bastida A, Meza-Nieto MA, Valladares-Carranza B, A. Alsayegh A, El-Saber Batiha G, Rivero-Perez N. nor 3′-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis. Molecules. 2022; 27(11):3620. https://doi.org/10.3390/molecules27113620
Chicago/Turabian StyleMorales-Ubaldo, Ana Lizet, Manases Gonzalez-Cortazar, Adrian Zaragoza-Bastida, Martín A. Meza-Nieto, Benjamín Valladares-Carranza, Abdulrahman A. Alsayegh, Gaber El-Saber Batiha, and Nallely Rivero-Perez. 2022. "nor 3′-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis" Molecules 27, no. 11: 3620. https://doi.org/10.3390/molecules27113620
APA StyleMorales-Ubaldo, A. L., Gonzalez-Cortazar, M., Zaragoza-Bastida, A., Meza-Nieto, M. A., Valladares-Carranza, B., A. Alsayegh, A., El-Saber Batiha, G., & Rivero-Perez, N. (2022). nor 3′-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis. Molecules, 27(11), 3620. https://doi.org/10.3390/molecules27113620