Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring
Abstract
:1. Introduction
2. Results
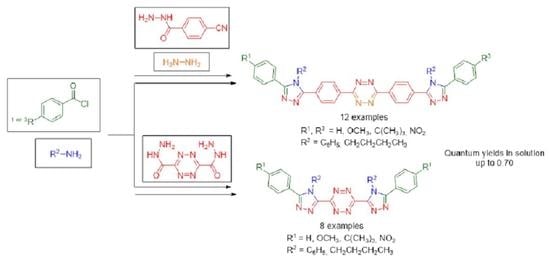
2.1. Synthesis
2.2. Luminescent Properties
3. Experimental Section
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. Synthesis of s-Tetrazine Derivatives Coupled via a 1,4-Phenylene Linkage with a 4H-1,2,4-Triazole Ring (10a–l)
3-(4-(4,5-Diphenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(5-(4-methoxyphenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10a)
3-(4-(5-(4-(tert-Butyl)phenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4,5-diphenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10b)
3-(4-(4,5-Diphenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(5-(4-nitrophenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10c)
3-(4-(5-(4-(tert-Butyl)phenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(5-(4-methoxyphenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10d)
3-(4-(5-(4-Methoxyphenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(5-(4-nitrophenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10e)
3-(4-(5-(4-(tert-Butyl)phenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(5-(4-nitrophenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10f)
3-(4-(4-Butyl-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10g)
3-(4-(4-Butyl-5-(4-(tert-butyl)phenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10h)
3-(4-(4-Butyl-5-(4-nitrophenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-phenyl-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10i)
3-(4-(4-Butyl-5-(4-(tert-butyl)phenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10j)
3-(4-(4-Butyl-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-(4-nitrophenyl)-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10k)
3-(4-(4-Butyl-5-(4-(tert-butyl)phenyl)-4H-1,2,4-triazol-3-yl)phenyl)-6-(4-(4-butyl-5-(4-nitrophenyl)-4H-1,2,4-triazol-3-yl)phenyl)-1,2,4,5-tetrazine (10l)
3.2.2. Synthesis of s-Tetrazine Derivatives Directly Conjugated with a 4H-1,2,4-Triazole Ring (15a–h)
3,6-Bis(4,5-diphenyl-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15a)
3,6-Bis(5-(4-methoxyphenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15b)
3,6-Bis(5-(4-(tert-butyl)phenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine(15c)
3,6-Bis(5-(4-nitrophenyl)-4-phenyl-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15d)
3,6-Bis(4-butyl-5-phenyl-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15e)
3,6-Bis(4-butyl-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15f)
3,6-Bis(4-butyl-5-(4-(tert-butyl)phenyl)-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15g)
3,6-Bis(4-butyl-5-(4-nitrophenyl)-4H-1,2,4-triazol-3-yl)-1,2,4,5-tetrazine (15h)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, T.; Zheng, C.; Yang, J.; Zhang, X.; Gong, X.; Xia, M. Theoretical studies on a new high energy density compound 6-amino-7-nitropyrazino[2,3-e][1,2,3,4]tetrazine-1,3,5-trioxide. J. Mol. Model. 2014, 20, 2261–2271. [Google Scholar] [CrossRef]
- Saracoglu, N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. Tetrahedron 2007, 63, 4199–4235. [Google Scholar] [CrossRef]
- Sinditskii, V.; Egorshev, V.Y.; Rudakov, G.F.; Filatov, S.A.; Burzhava, A.V. High-Nitrogen Energetic Materials of 1,2,4,5-Tetrazine Family: Thermal and Combustion Behaviors. In Chemical Rocket Propulsion a Comprehensive Survey of Energetic Materials; de Luca, L.T., Shimada, T., Sinditskii, V.P., Calabro, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 45, pp. 89–125. [Google Scholar] [CrossRef]
- Ishmetova, R.I.; Ignatenko, N.K.; Ganebnykh, I.N.; Tolschina, S.G.; Korotina, A.V.; Kravchenko, M.A.; Skornyakov, S.N.; Rusinov, G.L. Synthesis and tuberculostatic activity of amine-substituted 1,2,4,5-tetrazines and pyridazines. Russ. Chem. Bull. 2014, 63, 1423–1430. [Google Scholar] [CrossRef]
- Hu, W.X.; Rao, G.W.; Sun, Y.Q. Synthesis and antitumor activity of s-tetrazine derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Werbel, L.M.; Mc Namara, D.J.; Colbry, N.L.; Johnson, J.L.; Degan, M.J.; Whitney, B. Synthesis and antimalarial effects of N,N-dialkyl-6-(substituted phenyl)-1,2,4,5-tetrazin-3-amines. J. Heterocycl. Chem. 1979, 16, 881–894. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Upadhyay, R.; Haun, J.B.; Hilderbrand, S.A.; Weissleder, R. Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 7013–7016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Svatunek, D.; Rohlfing, K.; Liu, Y.; Wang, H.; Giglio, B.; Yuan, H.; Wu, Z.; Li, Z.; Fox, J. Conformationally strained trans-cyclooctene (sTCO) enables the rapid construction of 18F-PET probes via tetrazine ligation. Theranostics 2016, 6, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.P.; Smith, A.B. Peptide/protein stapling and unstapling: Introduction of s-tetrazine, photochemical release, and regeneration of the peptide/protein. J. Am. Chem. Soc. 2015, 137, 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, S.; Chen, P.R. Diels-Alder reaction–triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 2014, 10, 1003–1005. [Google Scholar] [CrossRef]
- Moral, M.; Garzon, A.; Olivier, Y.; Muccioli, L.; Sancho-Garcia, J.C.; Granadino-Roldan, J.M.; Fernandez-Gomez, M. Bis(arylene-ethynylene)-s-tetrazines: A promising family of n-type organic semiconductors? J. Phys. Chem. C 2015, 119, 18945–18955. [Google Scholar] [CrossRef] [Green Version]
- Pluczyk, S.; Zassowski, P.; Quinton, C.; Audebert, P.; Alain-Rizzo, V.; Łapkowski, M. Unusual electrochemical properties of the electropolymerized thin layer based on a s-tetrazine-triphenylamine monomer. J. Phys. Chem. C 2016, 120, 4382–4391. [Google Scholar] [CrossRef]
- Gaber, M.; Fathalla, S.K.; El-Ghamry, H.A. 2,4-Dihydroxy-5-[(5-mercapto-1H-1,2,4-triazole-3-yl)diazenyl]benzaldehyde acetato, chloro and nitrato Cu(II) complexes: Synthesis, structural characterization, DNA binding and anticancer and antimicrobial activity. Appl. Organomet. Chem. 2019, 33, e4707. [Google Scholar] [CrossRef]
- Shcherbyna, R.O.; Danilchenko, D.M.; Khromykh, N.O. The study of 2-((3-R-4-R1-4H-1,2,4-triazole-5-yl)thio) acetic acid salts as growth stimulators of winter wheat sprouts. Vìsn. Farm. 2017, 89, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Kaproń, B.; Łuszczki, J.J.; Płazińska, A.; Siwek, A.; Karcz, T.; Gryboś, A.; Nowak, G.; Makuch-Kocka, A.; Walczak, K.; Langner, E.; et al. Development of the 1, 2, 4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in-vivo, in-vitro, and in-silico studies. Eur. J. Pharm. Sci. 2019, 129, 42–57. [Google Scholar] [CrossRef]
- Jalihal, P.C.; Kashaw, V. Synthesis, antimicrobial and anti-inflammatory activity of some bioactive 1,2,4-triazoles analogues. Int. J. Pharm. Biol. Sci. 2018, 8, 94–104. [Google Scholar]
- Maindron, T.; Wang, Y.; Dodelet, J.P.; Miyatake, K.; Hlil, A.R.; Hay, A.S.; Tao, Y.; D’lorio, M. Highly electroluminescent devices made with a conveniently synthesized triazole-triphenylamine derivative. Thin Solid Film. 2004, 466, 209–216. [Google Scholar] [CrossRef]
- Dutta, R.; Kalita, D.J. Charge injection and hopping transport in bridged-dithiophene-triazole-bridged-dithiophene (DT-Tr-DT) conducting oligomers: A DFT approach. Comput. Theor. Chem. 2018, 1132, 42–49. [Google Scholar] [CrossRef]
- Tang, Y.; Zhuang, J.; Xie, L.; Chen, X.; Zhang, D.; Hao, J.; Su, W.; Cui, Z. Thermally cross-linkable host materials for solution-processed OLEDs: Synthesis, characterization, and optoelectronic properties. Eur. J. Org. Chem. 2016, 22, 3737–3747. [Google Scholar] [CrossRef]
- Tsai, L.R.; Yun, C. Hyperbranched luminescent polyfluorenes containing aromatic triazole branching units. J. Polym. Sci. A Polym. Chem. 2007, 45, 4465–4476. [Google Scholar] [CrossRef]
- Curtis, N.J.; Jennings, N. 1,2,4-Triazoles. In Comprehensive Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 5, pp. 159–209. [Google Scholar] [CrossRef]
- Clavier, G.; Audebert, P. s-Tetrazines as building blocks for new functional molecules and molecular materials. Chem. Rev. 2010, 110, 3299–3314. [Google Scholar] [CrossRef]
- Savastano, M.; García-Gallarín, C.; Dolores López de la Torre, M.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Anion-π and lone pair-π interactions with s-tetrazine-based ligands. Coord. Chem. Rev. 2019, 397, 112–137. [Google Scholar] [CrossRef]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Highly fluorescent 1,2,4,5-tetrazine derivatives containing 1,3,4-oxadiazolering conjugated via a 1,4-phenylene linker. Dyes Pigm. 2020, 183, 108715–108723. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Świątkowski, M. 1,3,4-Thiadiazol-2-ylphenyl-1,2,4,5-tetrazines: Efficient synthesis via Pinner reaction and their luminescent properties. Arkivoc 2021, 8, 167–178. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Świątkowski, M. Novel conjugated s-tetrazinederivatives bearing a 4H-1,2,4-triazole scaffold: Synthesis and luminescent properties. Molecules 2022, 27, 459. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dyes Pigm. 2020, 172, 107865–107872. [Google Scholar] [CrossRef]
- Fan, X.; Ge, Y.; Lin, F.; Yang, Y.; Zhang, G.; Ngai, W.S.C.; Lin, Z.; Zheng, S.; Wang, J.; Zhao, J.; et al. Optimized tetrazine derivatives for rapid bioorthogonal decaging in living cells. Angew. Chem. Int. Ed. 2016, 55, 14046–14050. [Google Scholar] [CrossRef]
- Yang, J.; Karver, M.R.; Li, W.; Sahu, S.; Devaraj, N.K. Metal-catalyzed one-pot synthesis of tetrazines directly from aliphatic nitriles and hydrazine. Angew. Chem. Int. Ed. 2012, 51, 5222–5225. [Google Scholar] [CrossRef]
- Chowdhury, M.; Goodman, L. Fluorescence of s-tetrazine. J. Chem. Phys. 1962, 36, 548–549. [Google Scholar] [CrossRef]
- Chowdhury, M.; Goodman, L. Nature of s-tetrazine emission spectra. J. Chem. Phys. 1963, 38, 2979–2985. [Google Scholar] [CrossRef]
- Choi, S.-K.; Kim, J.; Kim, E. Overview of syntheses and molecular-design strategies for tetrazine-based fluorogenic probes. Molecules 2021, 26, 1868. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. The coordination chemistry of Zn(II), Cd(II) and Hg(II) complexes with 1,2,4-triazole derivatives. Dalton Trans. 2011, 40, 8475–8490. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ge, H.; Yin, B.; She, M.; Liu, P.; Lia, X.; Li, J. Novel 3,6-unsymmetrically disubstituted-1,2,4,5-tetrazines: S-induced one-pot synthesis, properties and theoretical study. RSC Adv. 2015, 5, 12277–12886. [Google Scholar] [CrossRef]
- Gong, Y.-H.; Miomandre, F.; Méallet-Renault, R.; Badré, S.; Galmiche, L.; Tang, J.; Audebert, P.; Clavier, G. Synthesis and physical chemistry of s-tetrazines: Which ones are fluorescent and why? Eur. J. Org. Chem. 2009, 6121–6128. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Melhuish, W.H. Quantum efficiencies of fluorescence of organic substances: Effect of solvent and concentration of the fluorescent solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Birks, J.B.; Dyson, D.J. The relations between the fluorescence and absorption properties of organic molecules. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 275, 135–148. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, M. S1 (n,π*), T1 (n,π*) and S2 (n,π*) emissions in 3,6-diphenyl-s-tetrazine. Chem. Phys. Lett. 1982, 85, 233–238. [Google Scholar] [CrossRef]






| Entry | Product | R1 | R3 | R2 | Activating Agent | Yield [%] |
|---|---|---|---|---|---|---|
| 1 | 10a | H | OCH3 | Ph | S | 42 |
| 2 | Zn(CF3SO3)2 | 56 | ||||
| 3 | 10b | H | t-Bu | Ph | Zn(CF3SO3)2 | 52 |
| 4 | 10c | H | NO2 | Ph | Zn(CF3SO3)2 | 49 |
| 5 | 10d | OCH3 | t-Bu | Ph | Zn(CF3SO3)2 | 56 |
| 6 | 10e | OCH3 | NO2 | Ph | Zn(CF3SO3)2 | 54 |
| 7 | 10f | t-Bu | NO2 | Ph | Zn(CF3SO3)2 | 52 |
| 8 | 10g | H | OCH3 | n-Bu | S | 35 |
| 9 | Zn(CF3SO3)2 | 50 | ||||
| 10 | 10h | H | t-Bu | n-Bu | Zn(CF3SO3)2 | 47 |
| 11 | 10i | H | NO2 | n-Bu | Zn(CF3SO3)2 | 45 |
| 12 | 10j | OCH3 | t-Bu | n-Bu | Zn(CF3SO3)2 | 51 |
| 13 | 10k | OCH3 | NO2 | n-Bu | Zn(CF3SO3)2 | 48 |
| 14 | 10l | t-Bu | NO2 | n-Bu | Zn(CF3SO3)2 | 47 |
| Entry | Product | R1 | R2 | Yield [%] |
|---|---|---|---|---|
| 1 | 15a | H | Ph | 45 |
| 2 | 15b | OCH3 | Ph | 68 |
| 3 | 15c | t-Bu | Ph | 59 |
| 4 | 15d | NO2 | Ph | 40 |
| 5 | 15e | H | n-Bu | 56 |
| 6 | 15f | OCH3 | n-Bu | 78 |
| 7 | 15g | t-Bu | n-Bu | 73 |
| 8 | 15h | NO2 | n-Bu | 42 |
| Entry | Compound | λabs (nm) | ε | λex (nm) | λem (nm) | Stokes Shift (nm) | Φ | |
|---|---|---|---|---|---|---|---|---|
| (mol−1 dm3 cm−1) | qn-SO42− | dpb | ||||||
| 1 | 10a | 283 | 43,774 | 295 | 386 | 103 | 0.50 | 0.49 |
| 2 | 10b | 284 | 43,560 | 300 | 382 | 98 | 0.70 | 0.69 |
| 3 | 10c | 293 | 50,920 | 294 | 375 | 82 | 0.24 | 0.24 |
| 4 | 10d | 287 | 41,880 | 302 | 391 | 104 | 0.67 | 0.66 |
| 5 | 10e | 303 | 48,280 | 303 | 409 | 106 | 0.14 | 0.14 |
| 6 | 10f | 292 | 44,760 | 299 | 384 | 92 | 0.29 | 0.28 |
| 7 | 10g | 242 | 32,860 | 288 | 399 | 157 | 0.19 | 0.19 |
| 8 | 10h | 232 | 32,680 | 291 | 378 | 146 | 0.20 | 0.20 |
| 9 | 10i | 236 | 21,760 | 291 | 375 | 139 | 0.04 | 0.04 |
| 10 | 10j | 253 | 38,120 | 288 | 396 | 143 | 0.05 | 0.05 |
| 11 | 10k | 257 | 32,260 | 304 | 412 | 155 | 0.03 | 0.03 |
| 12 | 10l | 239 | 36,940 | 296 | 386 | 147 | 0.22 | 0.21 |
| 13 | 15a | 278 | 30,180 | 297 | 354 | 76 | 0.26 | 0.26 |
| 14 | 15b | 256 | 36,160 | 309 | 375 | 119 | 0.26 | 0.25 |
| 15 | 15c | 276 | 32,600 | 298 | 362 | 86 | 0.30 | 0.29 |
| 16 | 15d | 298 | 31,300 | - | - | - | - | - |
| 17 | 15e | 257 | 27,900 | 270 | 353 | 96 | 0.07 | 0.07 |
| 18 | 15f | 253 | 37,100 | 284 | 373 | 120 | 0.21 | 0.20 |
| 19 | 15g | 259 | 15,100 | 283 | 361 | 102 | 0.11 | 0.10 |
| 20 | 15h | 269 | 12,800 | - | - | - | - | - |
| Entry | R1 | R3 | Oxadiazole | Thiadiazole | Triazole R2 = Ph | Triazole R2 = n-Bu |
|---|---|---|---|---|---|---|
| 1 | H | H | 0.09 | 0.46 | 0.69 | 0.59 |
| 2 | OCH3 | OCH3 | 0.39 | 0.60 | >0.98 | 0.49 |
| 3 | t-Bu | t-Bu | 0.43 | 0.58 | 0.33 | 0.51 |
| 4 | NO2 | NO2 | 0.09 | 0.14 | 0.02 | 0.02 |
| 5 | H | OCH3 | 0.41 | 0.44 | 0.50 | 0.19 |
| 6 | H | t-Bu | 0.51 | 0.40 | 0.70 | 0.20 |
| 7 | H | NO2 | 0.57 | 0.26 | 0.24 | 0.04 |
| 8 | OCH3 | t-Bu | 0.54 | 0.53 | 0.67 | 0.05 |
| 9 | OCH3 | NO2 | 0.39 | 0.38 | 0.14 | 0.03 |
| 10 | t-Bu | NO2 | 0.05 | 0.26 | 0.29 | 0.22 |
| Entry | R1 | Oxadiazole | Thiadiazole | Triazole R2 = Ph | Triazole R2 = n-Bu |
|---|---|---|---|---|---|
| 1 | H | 0.10 | 0.74 | 0.26 | 0.07 |
| 2 | OCH3 | >0.98 | >0.98 | 0.26 | 0.21 |
| 3 | t-Bu | * | * | 0.30 | 0.11 |
| 4 | NO2 | 0.08 | 0.50 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, A.; Kudelko, A.; Świątkowski, M. Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring. Molecules 2022, 27, 3642. https://doi.org/10.3390/molecules27113642
Maj A, Kudelko A, Świątkowski M. Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring. Molecules. 2022; 27(11):3642. https://doi.org/10.3390/molecules27113642
Chicago/Turabian StyleMaj, Anna, Agnieszka Kudelko, and Marcin Świątkowski. 2022. "Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring" Molecules 27, no. 11: 3642. https://doi.org/10.3390/molecules27113642
APA StyleMaj, A., Kudelko, A., & Świątkowski, M. (2022). Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring. Molecules, 27(11), 3642. https://doi.org/10.3390/molecules27113642