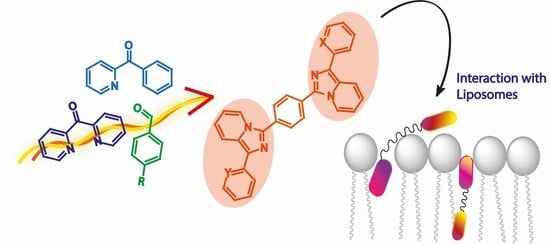
Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Optical Features
2.3. Liposomes
3. Materials and Methods
3.1. General Methods
3.2. Synthetic Procedures
3.3. Optical Features
3.4. Experiments with Liposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Volpi, G.; Rabezzana, R. Imidazo[1,5-a]pyridine derivatives: Useful, luminescent and versatile scaffolds for different applications. New J. Chem. 2021, 45, 5737–5743. [Google Scholar] [CrossRef]
- Colombo, G.; Ardizzoia, G.A.; Brenna, S. Imidazo[1,5-a]pyridine-based derivatives as highly fluorescent dyes. Inorg. Chim. Acta 2022, 535, 120849. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Garino, C.; Barolo, C.; Costa, R.D. Contextualizing yellow light-emitting electrochemical cells based on a blue-emitting imidazo-pyridine emitter. Polyhedron 2018, 140, 129–137. [Google Scholar] [CrossRef]
- Volpi, G. Luminescent imidazo[1,5-a]pyridine scaffold: Synthetic heterocyclization strategies overview and promising applications. Asian J. Org. Chem. 2022. [Google Scholar] [CrossRef]
- Wang, J.; Dyers, L.; Mason, R.; Amoyaw, P.; Bu, X.R. Highly Efficient and Direct Heterocyclization of Dipyridyl Ketone to N,N-Bidentate Ligands. J. Org. Chem. 2005, 70, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Wang, Z.; Yu, L.; Xu, K. Copper-Catalyzed Oxidative Amination of sp3 C–H Bonds under Air: Synthesis of 1,3-Diarylated Imidazo[1,5-a]pyridines. J. Org. Chem. 2015, 80, 2431–2435. [Google Scholar] [CrossRef]
- Shibahara, F.; Yamaguchi, E.; Kitagawa, A.; Imai, A.; Murai, T. Synthesis of 1,3-diarylated imidazo[1,5-a]pyridines with a combinatorial approach: Metal-catalyzed cross-coupling reactions of 1-halo-3-arylimidazo[1,5-a]pyridines with arylmetal reagents. Tetrahedron 2009, 65, 5062–5073. [Google Scholar] [CrossRef]
- Roy, M.; Chakravarthi BV, S.K.; Jayabaskaran, C.; Karande, A.A.; Chakravarty, A.R. Impact of metal binding on the antitumor activity and cellular imaging of a metal chelator cationic imidazopyridine derivative. Dalton Trans. 2011, 40, 4855–4864. [Google Scholar] [CrossRef]
- Priyanga, S.; Khamrang, T.; Velusamy, M.; Karthi, S.; Ashokkumar, B.; Mayilmurugan, R. Coordination geometry-induced optical imaging of L-cysteine in cancer cells using imidazopyridine-based copper(II) complexes. Dalton Trans. 2019, 48, 1489–1503. [Google Scholar] [CrossRef]
- Volpi, G.; Priola, E.; Garino, C.; Daolio, A.; Rabezzana, R.; Benzi, P.; Giordana, A.; Diana, E.; Gobetto, R. Blue fluorescent zinc(II) complexes based on tunable imidazo[1,5-a]pyridines. Inorg. Chim. Acta 2020, 509, 119662–119671. [Google Scholar] [CrossRef]
- Guckian, A.L.; Doering, M.; Ciesielski, M.; Walter, O.; Hjelm, J.; O’ Boyle, N.M.; Henry, W.; Browne, W.R.; McGarvey, J.J.; Vos, J.G. Assessment of intercomponent interaction in phenylene bridged dinuclear ruthenium(ii) and osmium(ii) polypyridyl complexes. Dalton Trans. 2004, 23, 3943–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chen, Z.; Liu, A.; Ji, R.; Cao, X.; Ge, Y. A ratiometric fluorescent probe for detection of exogenous mitochondrial SO2 based on a FRET mechanism. RSC Adv. 2019, 9, 8943–8948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Zhang, L.; Zhou, Z.; Wang, S.; Xu, Y.; Gu, Y.; Zha, X. A new fluorescent probe for quick and highly selective detection of hydrogen sulfide and its application in living cells. New J. Chem. 2018, 42, 13884–13888. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Milanesio, M.; Garino, C.; Barolo, C.; Costa, R.D. Novel Ligand and Device Designs for Stable Light-Emitting Electrochemical Cells Based on Heteroleptic Copper(I) Complexes. Inorg. Chem. 2018, 57, 10469–10479. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, F.; Tanigawa, J.; Nii, C.; Tabata, A.; Nagamune, H.; Takanari, H.; Imada, Y.; Kawamura, Y. Fluorescent Imidazo[1,5-a]pyridinium Salt for a Potential Cancer Therapy Agent. ACS Med. Chem. Lett. 2019, 10, 1110–1114. [Google Scholar] [CrossRef]
- Song, G.J.; Bai, S.Y.; Dai, X.; Cao, X.Q.; Zhao, B.X. A ratiometric lysosomal pH probe based on the imidazo[1,5-a]pyridine–rhodamine FRET and ICT system. RSC Adv. 2016, 6, 41317–41322. [Google Scholar] [CrossRef]
- Volpi, G.; Lace, B.; Garino, C.; Priola, E.; Artuso, E.; Cerreia Vioglio, P.; Barolo, C.; Fin, A.; Genre, A.; Prandi, C. New substituted imidazo[1,5-a]pyridine and imidazo[5,1-a]isoquinoline derivatives and their application in fluorescence cell imaging. Dyes Pigm. 2018, 157, 298–304. [Google Scholar] [CrossRef]
- Sitarska, E.; Munoz, A.D. Pay attention to membrane tension: Mechanobiology of the cell surface. Curr. Opin. Cell Biol. 2020, 66, 11–18. [Google Scholar] [CrossRef]
- Leiphart, R.J.; Chen, D.; Peredo, A.P.; Loneker, A.E.; Janmey, P.A. Mechanosensing at cellular interfaces. Langmuir 2019, 35, 7509–7519. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Weber, G.; Farris, F.J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6 propionyl-2-(dimethylamino)naphthalene. Biochemistry 1979, 18, 3075–3078. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Bevan, D.R.; Maliwal, B.P.; Cherek, H.; Balter, A. Synthesis and characterization of a fluorescence probe of the phase transition and dynamic properties of membranes. Biochemistry 1983, 22, 5714–5722. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.R.; Barenholz, Y.; Thompson, T.E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry 1976, 15, 4521. [Google Scholar] [CrossRef] [PubMed]
- Badley, R.A.; Schneider, H.; Martin, W.G. Dynamic behavior of fluorescent probes in lipid bilayer model membranes. Biochemistry 1973, 12, 268. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorg. Chem. 2005, 33, 415. [Google Scholar] [CrossRef]
- Invitrogen Corporation, Molecular Probes—The Handbook. Available online: https://www.invitrogen.com/site/us/en/home/References/Molecular-ProbesThe-Handbook.html (accessed on 24 November 2009).
- Lapinski, M.M.; Blanchard, G.J. The role of phospholipid headgroups in mediating bilayer organization. Perturbations induced by the presence of a tethered chromophore. Chem. Phys. Lipids 2007, 150, 12–21. [Google Scholar] [CrossRef]
- Waggoner, A.S.; Stryer, L. Fluorescent Probes of Biological Membranes. Proc. Natl. Acad. Sci. USA 1970, 67, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.E.; Lentz, B.R. Phospholipid lateral organization in synthetic membranes as monitored by pyrene-labeled phospholipids: Effects of temperature and prothrombin fragment 1 binding. Biochemistry 1986, 25, 567–574. [Google Scholar] [CrossRef]
- Starck, J.P.; Nakatani, Y.; Ourisson, G. Synthesis of two new phospholipidic fluorescent probes for membrane studies. Tetrahedron 1995, 51, 2629–2638. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Ashokkumar, P.; Kovtun, Y.P.; Klymchenko, A.S. Solvatochromic Near-Infrared probe for polarity mapping of biomembranes and lipid droplets in cells under stress. J. Phys. Chem. Lett. 2019, 10, 2414–2421. [Google Scholar] [CrossRef] [Green Version]
- García-Calvo, J.; López-Andarias, J.; Maillard, J.; Mercier, V.; Roffay, C.; Roux, A.; Fürstenberg, A.; Sakai, N.; Matile, S. HydroFlipper membrane tension probes: Imaging membrane hydration and mechanical compression simultaneously in living cells. Chem. Sci. 2022, 13, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Magnano, G.; Benesperi, I.; Saccone, D.; Priola, E.; Gianotti, V.; Milanesio, M.; Conterosito, E.; Barolo, C.; Viscardi, G. One pot synthesis of low cost emitters with large Stokes’ shift. Dyes Pigm. 2017, 137, 152–164. [Google Scholar] [CrossRef]
- Wang, J.; Mason, R.; VanDerveer, D.; Feng, K.; Bu, X.R. Convenient Preparation of a Novel Class of Imidazo[1,5-a]pyridines: Decisive Role by Ammonium Acetate in Chemoselectivity. J. Org. Chem. 2003, 68, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Garino, C.; Priola, E.; Magistris, C.; Chierotti, M.R.; Barolo, C. Halogenated imidazo[1,5-a] pyridines: Chemical structure and optical properties of a promising luminescent scaffold. Dyes Pigm. 2019, 171, 107713. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Priola, E.; Diana, E.; Gobetto, R.; Buscaino, R.; Viscardi, G.; Barolo, C. Facile synthesis of novel blue light and large Stoke shift emitting tetradentate polyazines based on imidazo[1,5-a]pyridine e Part 2. Dyes Pigm. 2017, 143, 284–290. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Conterosito, E.; Barolo, C.; Gobetto, R.; Viscardi, G. Facile synthesis of novel blue light and large Stoke shift emitting tetradentate polyazines based on imidazo[1,5-a]pyridine. Dyes Pigm. 2016, 128, 96–100. [Google Scholar] [CrossRef]
- Volpi, G.; Galliano, S.; Buscaino RViscardi, G.; Barolo, C. Fluorescent trifluoromethylated imidazo[1,5-a]pyridines and their application in luminescent down-shfiting conversion. J. Lumin. 2022, 2442, 118529. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Albrecht, G.; Rossiger, C.; Herr, J.M.; Locke, H.; Yanagi, H.; Gottlich, R.; Schlettwein, D. Optimization of the substitution pattern of 1,3-disubstituted imidazo[1,5-a]Pyridines and -quinolines for electro-optical applications. Phys. Status Solidi B 2020, 257, 1900677–1900687. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Haugland, R.P. Partition coefficients of fluorescent probes with phospholipid membranes. Biochem. Biophys. Res. Commun. 1991, 181, 166–171. [Google Scholar] [CrossRef]







| Compound | Solvent | λabs [nm] | ε [M−1 cm−1] | λem [nm] | Stokes Shift [cm−1 (nm)] | QY a |
|---|---|---|---|---|---|---|
| 1 | Dioxane | 300 | 17,430 | 482 | 12,586 (182) | 0.10 |
| DMF | 288 | 16,280 | 480 | 13,889 (192) | 0.13 | |
| Ethyl Acetate | 302 | 12,980 | 480 | 12,279 (178) | 0.14 | |
| Methanol | 293 | 12,770 | 481 | 13,340 (188) | 0.13 | |
| THF | 301 | 10,580 | 479 | 12,346 (178) | 0.17 | |
| Toluene | 305 | 12,315 | 482 | 12,040 (177) | 0.20 | |
| 2 | Dioxane | 377 | 29,066 | 488 | 6033 (111) | 0.11 |
| DMF | 379 | 26,470 | 479 | 5508 (100) | 0.08 | |
| Ethyl Acetate | 375 | 30,205 | 484 | 6006 (109) | 0.08 | |
| Methanol | 370 | 23,960 | 474 | 5930 (104) | 0.10 | |
| THF | 379 | 23,910 | 484 | 5724 (105) | 0.11 | |
| Toluene | 382 | 23,810 | 486 | 5602 (104) | 0.12 | |
| 3 | Dioxane | 376 | 23,760 | 483 | 5892 (107) | 0.13 |
| DMF | 377 | 17,430 | 471 | 5294 (94) | 0.12 | |
| Ethyl Acetate | 375 | 22,130 | 478 | 5746 (103) | 0.11 | |
| Methanol | 368 | 16,305 | 464 | 5622 (96) | 0.08 | |
| THF | 374 | 13,180 | 470 | 5461 (96) | 0.09 | |
| Toluene | 378 | 16,820 | 480 | 5622 (102) | 0.18 | |
| 4 | Dioxane | 380 | 33,950 | 478 | 5395 (98) | 0.29 |
| DMF | 384 | 28,660 | 451 | 3869 (67) | 0.17 | |
| Ethyl Acetate | 379 | 33,795 | 472 | 5199 (93) | 0.24 | |
| Methanol | 369 | 27,475 | 460 | 5361 (93) | 0.15 | |
| THF | 384 | 30,248 | 482 | 5295 (98) | 0.26 | |
| Toluene | 383 | 28,922 | 470 | 4833 (87) | 0.38 | |
| 5 | Dioxane | 322 | 28,980 | 460 | 9317 (138) | 0.13 |
| DMF | 322 | 19,240 | 461 | 9364 (139) | 0.18 | |
| Ethyl Acetate | 326 | 24,707 | 459 | 8888 (133) | 0.16 | |
| Methanol | 313 | 18,270 | 457 | 10,067 (144) | 0.16 | |
| THF | 319 | 18,138 | 460 | 9609 (141) | 0.19 | |
| Toluene | 325 | 20,745 | 461 | 9077 (136) | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renno, G.; Cardano, F.; Volpi, G.; Barolo, C.; Viscardi, G.; Fin, A. Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models. Molecules 2022, 27, 3856. https://doi.org/10.3390/molecules27123856
Renno G, Cardano F, Volpi G, Barolo C, Viscardi G, Fin A. Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models. Molecules. 2022; 27(12):3856. https://doi.org/10.3390/molecules27123856
Chicago/Turabian StyleRenno, Giacomo, Francesca Cardano, Giorgio Volpi, Claudia Barolo, Guido Viscardi, and Andrea Fin. 2022. "Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models" Molecules 27, no. 12: 3856. https://doi.org/10.3390/molecules27123856
APA StyleRenno, G., Cardano, F., Volpi, G., Barolo, C., Viscardi, G., & Fin, A. (2022). Imidazo[1,5-a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models. Molecules, 27(12), 3856. https://doi.org/10.3390/molecules27123856