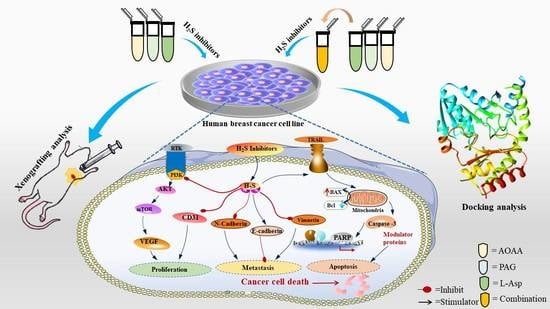
Pharmacological Inhibition of Endogenous Hydrogen Sulfide Attenuates Breast Cancer Progression
Abstract
:1. Introduction
2. Material Method
2.1. Cell Culture
2.2. Drugs Formulations/Treatment
2.3. Cell Viability Assay
2.4. Detection of H2S Level
2.5. Cell Proliferation Assay
2.6. Wound Healing Assay
2.7. Colony Formation Assay
2.8. Migration and Invasion Assay
2.9. Cell Death Assay
2.10. Western Blot Analysis
2.11. Animal Study
2.12. Tumor Tissue Staining
2.13. Immunohistochemistry (IHC)
2.14. Statistics Analysis
3. In silico Validation of Drugs/Inhibitors
3.1. Ligand and Receptor Protein Preparation
3.2. Druggable Pockets and Molecular Docking Analysis
4. Results
4.1. Inhibition of Endogenous H2S Attenuates the Viability and Proliferation of Human BC Cells
4.2. Inhibition of Endogenous H2S Reduces the Migration and Invasion Rate of Human BC Cells
4.3. Suppression of Endogenous H2S Induces Apoptosis in Human BC Cells
4.4. Suppression of Endogenous H2S Inhibits Epithelial–Mesenchymal Transition in Human BC Cells
4.5. Suppression of Endogenous H2S Disrupts the PI3K/AKT/mTOR Pathway in Human BC Cells
4.6. Suppression of Endogenous H2S Inhibits the Angiogenesis and Growth of Human BC Xenograft Tumors
4.7. Molecular Docking Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fleege, N.M.; Cobain, E.F. Breast Cancer Management in 2021: A Primer for the OB GYN. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, in press. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Lubinski, J.; Lynch, H.T.; Ghadirian, P.; Foulkes, W.; Kim-Sing, C.; Neuhausen, S.; Tung, N.; Rosen, B.; Gronwald, J.; et al. Family History of Cancer and Cancer Risks in Women with BRCA1 or BRCA2 Mutations. JNCI J. Natl. Cancer Inst. 2010, 102, 1874–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britt, K.L.; Cuzick, J.; Phillips, K.-A. Key steps for effective breast cancer prevention. Nat. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef]
- Khan, N.H.; Duan, S.-F.; Wu, D.-D.; Ji, X.-Y. Better Reporting and Awareness Campaigns Needed for Breast Cancer in Pakistani Women. Cancer Manag. Res. 2021, 13, 2125–2129. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2017, 118, 1253–1337. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous Hydrogen Sulfide Production Is Essential for Dietary Restriction Benefits. Cell 2014, 160, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Chen, S.; Tang, C.; Wang, G.; Du, J.; Jin, H. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J. Adv. Res. 2020, 27, 19–30. [Google Scholar] [CrossRef]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, C. Hydrogen Sulfide, an Endogenous Stimulator of Mitochondrial Function in Cancer Cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular Functions of Hydrogen Sulfide in Cancer. Pathophysiology 2021, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.-F.; Xu, H.-R.; Yu, S.-H.; Li, P.; Lu, Y.-Y.; Chen, J.; Bi, Z.-Q.; Sun, H.-S.; Cheng, J.; Zhuang, H.-Q.; et al. ADT-OH inhibits malignant melanoma metastasis in mice via suppressing CSE/CBS and FAK/Paxillin signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Yang, B.; Han, J.-G.; Zhang, M.-M.; Liu, W.; Zhang, X.; Yu, H.-L.; Liu, Z.-G.; Zhang, S.-H.; Li, T.; et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019, 455, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sonke, E.; Verrydt, M.; Postenka, C.O.; Pardhan, S.; Willie, C.J.; Mazzola, C.R.; Hammers, M.D.; Pluth, M.D.; Lobb, I.; Power, N.E.; et al. Inhibition of endogenous hydrogen sulfide production in clear-cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential. Nitric Oxide 2015, 49, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yue, T.; Huang, Z.; Zhu, J.; Bu, D.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019, 466, 49–60. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Chen, S.; Wen, J.Y.; Chen, Z.W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endo-thelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol. Cell Physiol. 2020, 319, C720–C733. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.; Chao, C.; Módis, K.; Ding, Y.; Zatarain, J.; Thanki, K.; Maskey, M.; Druzhyna, N.; Untereiner, A.; Ahmad, A.; et al. Efficacy of Novel Aminooxyacetic Acid Prodrugs in Colon Cancer Models: Towards Clinical Translation of the Cystathionine β-Synthase Inhibition Concept. Biomolecules 2021, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Islam, M.; Prieto, M.; Majid, D. Interdependency of cystathione gam-ma-lyase and cystathione beta-synthase in hydrogen sulfide-induced blood pressure regulation in rats. Am. J. Hypertens. 2011, 25, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Ihara, E.; Hirano, K.; Tanaka, Y.; Nakano, K.; Kita, S.; Ogawa, Y. Endogenous hydrogen sulfide contributes to tone generation in porcine lower esophageal sphincter via Na+/Ca2+ exchanger. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, T.; Zuo, S.; Bu, D.; Zhu, J.; Chen, S.; Ma, Y.; Ma, J.; Guo, S.; Wen, L.; Zhang, X.; et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J. Cancer 2020, 11, 1828–1838. [Google Scholar] [CrossRef]
- Wu, D.; Li, M.; Tian, W.; Wang, S.; Cui, L.; Li, H.; Wang, H.; Ji, A.; Li, Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017, 7, 5134. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhong, P.; Wang, Y.; Zhang, Q.; Li, J.; Liu, Z.; Ji, A.; Li, Y. Hydrogen sulfide attenuates high-fat diet-induced non-alcoholic fatty liver disease by inhibiting apoptosis and promoting autophagy via reactive oxygen species/phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling pathway. Front. Pharmacol. 2020, 11, 585860. [Google Scholar] [CrossRef]
- Wu, D.; Li, M.; Gao, Y.; Tian, W.; Li, J.; Zhang, Q.; Liu, Z.; Zheng, M.; Wang, H.; Wang, J.; et al. Peptide V3 Inhibits the Growth of Human Hepatocellular Carcinoma by Inhibiting the Ras/Raf/MEK/ERK Signaling Pathway. J. Cancer 2019, 10, 1693–1706. [Google Scholar] [CrossRef]
- Dong, P.; Fu, H.; Chen, L.; Zhang, S.; Zhang, X.; Li, H.; Wu, D.; Ji, X. PCNP promotes ovarian cancer progression by accelerating β-catenin nuclear accumulation and triggering EMT transition. J. Cell. Mol. Med. 2020, 24, 8221–8235. [Google Scholar] [CrossRef]
- Mbagwu, S.I.; Filgueira, L. Differential Expression of CD31 and Von Willebrand Factor on Endothelial Cells in Different Regions of the Human Brain: Potential Implications for Cerebral Malaria Pathogenesis. Brain Sci. 2020, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Lanng, M.B.; Møller, C.B.; Andersen, A.-S.H.; Pálsdóttir, A.; Røge, R.; Østergaard, L.R.; Jørgensen, A.S. Quality assessment of Ki67 staining using cell line proliferation index and stain intensity features. Cytom. Part A 2018, 95, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Masumoto, J.; Tada, T.; Nomiyama, T.; Hongo, K.; Nakayama, J. Prognostic significance of the immunohisto-chemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin. Cancer Res. 2007, 13, 3868–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, A.; Tan, Y.-C.; Shahid, M.; Yow, Y.-Y.; Lahiri, C. Metabolite Profiling of Malaysian Gracilaria edulis Reveals Eplerenone as Novel Antibacterial Compound for Drug Repurposing Against MDR Bacteria. Front. Microbiol. 2021, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Zhang, W.D.; Jin, H.Z.; Basha, S.H.; Priya, S.S. In-silico anti-inflammatory potential of guaiane dimers from Xylo-pia vielana targeting COX-2. J. Biomol. Struct. Dyn. 2022, 40, 484–498. [Google Scholar] [CrossRef]
- Azfaralariff, A.; Farahfaiqah, F.; Shahid, M.; Sanusi, S.A.; Law, D.; Isa, A.R.M.; Muhamad, M.; Tsui, T.T.; Fazry, S. Marantodes pumilum: Systematic computational approach to identify their therapeutic potential and effectiveness. J. Ethnopharmacol. 2021, 283, 114751. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Shams ul Hassan, S.; Abbas, S.Q.; Hassan, M.; Jin, H.Z. Computational Exploration of Anti-Cancer Potential of GUAIANE Dimers from Xylopia vielana by Targeting B-Raf Kinase Using Chemo-Informatics, Molecular Docking, and MD Simulation Studies. Anti-Cancer Agents Med. Chem. (Former Curr. Med. Chem. Anti-Cancer Agents) 2022, 22, 731–746. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.-D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2015, 16, 7–21. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; VanDyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wu, X.; Kong, X.; Li, J.; Dong, C. RNF20 Is Critical for Snail-Mediated E-Cadherin Repression in Human Breast Cancer. Front. Oncol. 2020, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Zhang, Z.; Yao, H.; Shen, L. Tim-4 promotes the growth of colorectal cancer by activating angiogenesis and recruiting tumor-associated macrophages via the PI3K/AKT/mTOR signaling pathway. Cancer Lett. 2018, 436, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Ren, G.; Shi, Z.; Teng, C.; Yao, Y. Antiproliferative Activity of Combined Biochanin A and Ginsenoside Rh2 on MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 2908. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, X.; Liu, W.; Qin, X.; Hu, B.; Ma, Q.; Lv, C.; Lu, J. Cimicifuga dahurica extract inhibits the proliferation, migration and invasion of breast cancer cells MDA-MB-231 and MCF-7 in vitro and in vivo. J. Ethnopharmacol. 2021, 277, 114057. [Google Scholar] [CrossRef]
- Shahid, M.; Azfaralariff, A.; Law, D.; Najm, A.A.; Sanusi, S.A.; Lim, S.J.; Cheah, Y.H.; Fazry, S. Comprehensive computational target fishing approach to identify Xanthorrhizol putative targets. Sci. Rep. 2021, 11, 1594. [Google Scholar] [CrossRef]
- Yuan, A.; Hao, C.; Wu, X.; Sun, M.; Qu, A.; Xu, L.; Xu, C. Chiral CuxOS@ ZIF-8 Nanostructures for Ultrasensitive Quantification of Hydrogen Sulfide In Vivo. Adv. Mater. 2020, 32, 1906580. [Google Scholar] [CrossRef]
- Ngowi, E.E.; Afzal, A.; Sarfraz, M.; Khattak, S.; Zaman, S.U.; Khan, N.H.; Li, T.; Jiang, Q.-Y.; Zhang, X.; Duan, S.-F.; et al. Role of hydrogen sulfide donors in cancer development and progression. Int. J. Biol. Sci. 2021, 17, 73–88. [Google Scholar] [CrossRef]
- Ngowi, E.E.; Sarfraz, M.; Afzal, A.; Khan, N.H.; Khattak, S.; Zhang, X.; Li, T.; Duan, S.-F.; Ji, X.-Y.; Wu, D.-D. Roles of Hydrogen Sulfide Donors in Common Kidney Diseases. Front. Pharmacol. 2020, 11, 564281. [Google Scholar] [CrossRef] [PubMed]
- Ascenção, K.; Dilek, N.; Augsburger, F.; Panagaki, T.; Zuhra, K.; Szabo, C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H2S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacol. Res. 2021, 165, 105393. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug repurposing for breast cancer therapy: Old weapon for new battle. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Untereiner, A.; Pavlidou, A.; Druzhyna, N.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Drug resistance induces the upregulation of H2S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018, 149, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Collins, R.; Huang, S.; Holmberg-Schiavone, L.; Anand, G.S.; Tan, C.H.; Van-den-Berg, S.; Deng, L.W.; Moore, P.K.; Karlberg, T.; et al. Structural basis for the inhibition mechanism of human cystathi-onine γ-lyase, an enzyme responsible for the production of H2S. J. Biol. Chem. 2009, 284, 3076–3085. [Google Scholar] [CrossRef] [Green Version]
- Meier, M.; Janosik, M.; Kery, V.; Kraus, J.P.; Burkhard, P. Structure of human cystathionine β-synthase: A unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J. 2001, 20, 3910–3916. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S produc-tion by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Zhao, C.-C.; Yi, H.; Geng, Z.-J.; Wu, X.-Y.; Zhang, Y.; Liu, Y.; Fan, G. Traditional Tibetan Medicine in Cancer Therapy by Targeting Apoptosis Pathways. Front. Pharmacol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A Time to Kill: Targeting Apoptosis in Cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1α activation. Biochem. Pharmacol. 2019, 172, 113775. [Google Scholar] [CrossRef]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef]
- Sun, C.-H.; Chang, Y.-H.; Pan, C.-C. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology 2011, 58, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nie, H.; Zhao, X.; Qin, Y.; Gong, X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer 2016, 16, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Yang, H.; Zhang, Y.; Hu, R.; Hu, D.; Wang, Q.; Liu, Y.; Liu, M.; Meng, Z.; Zhou, W.; et al. Inhibition of cystathionine β-synthase promotes apoptosis and reduces cell proliferation in chronic myeloid leukemia. Signal Transduct. Target. Ther. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Cano-Galiano, A.; Oudin, A.; Fack, F.; Allega, M.F.; Sumpton, D.; Martinez-Garcia, E.; Niclou, S.P. Cystathionine-γ-lyase drives antioxidant defense in cysteine-restricted IDH1 mutant astrocytomas. Neuro-Oncol. Adv. 2021, 3, vdab057. [Google Scholar] [CrossRef] [PubMed]







| Drug | PubChem ID | Cystathionine Gamma-Lyase | 3-Mercaptopyruvate Sulfurtransferase | Cystathionine Beta-Synthase |
|---|---|---|---|---|
| L-aspartic acid | 5960 | −4.9 | −5.3 | −4.5 |
| DL-Propargylglycine | 95575 | −5.4 | −5.1 | −4.8 |
| Aminooxyacetic acid | 286 | −4 | −4.6 | −4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.H.; Wang, D.; Wang, W.; Shahid, M.; Khattak, S.; Ngowi, E.E.; Sarfraz, M.; Ji, X.-Y.; Zhang, C.-Y.; Wu, D.-D. Pharmacological Inhibition of Endogenous Hydrogen Sulfide Attenuates Breast Cancer Progression. Molecules 2022, 27, 4049. https://doi.org/10.3390/molecules27134049
Khan NH, Wang D, Wang W, Shahid M, Khattak S, Ngowi EE, Sarfraz M, Ji X-Y, Zhang C-Y, Wu D-D. Pharmacological Inhibition of Endogenous Hydrogen Sulfide Attenuates Breast Cancer Progression. Molecules. 2022; 27(13):4049. https://doi.org/10.3390/molecules27134049
Chicago/Turabian StyleKhan, Nazeer Hussain, Di Wang, Wenkang Wang, Muhammad Shahid, Saadullah Khattak, Ebenezeri Erasto Ngowi, Muhammad Sarfraz, Xin-Ying Ji, Chun-Yang Zhang, and Dong-Dong Wu. 2022. "Pharmacological Inhibition of Endogenous Hydrogen Sulfide Attenuates Breast Cancer Progression" Molecules 27, no. 13: 4049. https://doi.org/10.3390/molecules27134049
APA StyleKhan, N. H., Wang, D., Wang, W., Shahid, M., Khattak, S., Ngowi, E. E., Sarfraz, M., Ji, X. -Y., Zhang, C. -Y., & Wu, D. -D. (2022). Pharmacological Inhibition of Endogenous Hydrogen Sulfide Attenuates Breast Cancer Progression. Molecules, 27(13), 4049. https://doi.org/10.3390/molecules27134049