Redox-Active Metal-Organic Frameworks with Three-Dimensional Lattice Containing the m-Tetrathiafulvalene-Tetrabenzoate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structures
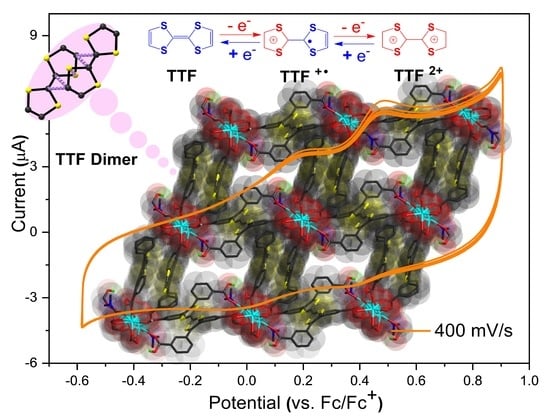
2.2. Cyclic Voltammetry
2.3. Absorption Spectra and Semiconducting Properties
2.4. Magnetic Properties
3. Materials and Methods
3.1. Materials and Methods
3.2. X-ray Structure
3.3. Synthesis of m-H4TTFTB
3.4. Synthesis of Dy-m-TTFTB, [Dy2(m-TTFTB)(m-H2TTFTB)0.5 (HCOO)(DMF)]·2DMF·3H2O
3.5. Synthesis of Tb-m-TTFTB, [Tb2(m-TTFTB)(m-H2TTFTB)0.5 (HCOO)(DMF)]·2DMF·3H2O
3.6. Synthesis of Er-m-TTFTB, [Er2(m-TTFTB)(m-H2TTFTB)0.5(HCOO)(DMF)]·2DMF·3H2O
3.7. Synthesis of Gd-m-TTFTB, [Gd2(m-TTFTB)(m-H2TTFTB)0.5(HCOO)(DMF)]·2DMF·3H2O
3.8. Solid CV
3.9. Solid-State Diffuse Reflectance Spectra
3.10. Electrical Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ding, B.; Solomon, M.B.; Leong, C.F.; D’Alessandro, D.M. Redox-active ligands: Recent advances towards their incorporation into coordination polymers and metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213891. [Google Scholar] [CrossRef]
- D’Alessandro, D.M. Exploiting redox activity in metal-organic frameworks: Concepts, trends and perspectives. Chem. Commun. 2016, 52, 8957–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Souto, M.; Strutynski, K.; Melle-Franco, M.; Rocha, J. Electroactive Organic Building Blocks for the Chemical Design of Functional Porous Frameworks (MOFs and COFs) in Electronics. Chem. Eur. J. 2020, 26, 10912–10935. [Google Scholar] [CrossRef] [PubMed]
- Usov, P.M.; Fabian, C.; D’Alessandro, D.M. Rapid determination of the optical and redox properties of a metal-organic framework via in situ solid state spectroelectrochemistry. Chem. Commun. 2012, 48, 3945–3947. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Wang, H.Y.; Huang, L.; Ge, J.Y.; Joseph, E.; Qin, J.; Cagin, T.; Zuo, J.L.; Zhou, H.C. Redox-switchable breathing behavior in tetrathiafulvalene-based metal-organic frameworks. Nat. Commun. 2017, 8, 2008. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, Y.M.; Gayfulin, Y.M.; Kovalenko, K.A.; Samsonenko, D.G.; van Leusen, J.; Korolkov, I.V.; Fedin, V.P.; Mironov, Y.V. Multifunctional Metal-Organic Frameworks Based on Redox-Active Rhenium Octahedral Clusters. Inorg. Chem. 2018, 57, 2072–2084. [Google Scholar] [CrossRef]
- Aubrey, M.L.; Wiers, B.M.; Andrews, S.C.; Sakurai, T.; Reyes-Lillo, S.E.; Hamed, S.M.; Yu, C.J.; Darago, L.E.; Mason, J.A.; Baeg, J.O.; et al. Electron delocalization and charge mobility as a function of reduction in a metal-organic framework. Nat. Mater. 2018, 17, 625–632. [Google Scholar] [CrossRef]
- Monni, N.; Oggianu, M.; Ashoka Sahadevan, S.; Mercuri, M.L. Redox Activity as a Powerful Strategy to Tune Magnetic and/or Conducting Properties in Benzoquinone-Based Metal-Organic Frameworks. Magnetochemistry 2021, 7, 109. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Cui, L.; Xie, J.-Z.; Leong, C.F.; D’Alessandro, D.M.; Zuo, J.-L. Functional coordination polymers based on redox-active tetrathiafulvalene and its derivatives. Coord. Chem. Rev. 2017, 345, 342–361. [Google Scholar] [CrossRef]
- Huo, P.; Chen, T.; Hou, J.L.; Yu, L.; Zhu, Q.Y.; Dai, J. Ligand-to-Ligand Charge Transfer within Metal-Organic Frameworks Based on Manganese Coordination Polymers with Tetrathiafulvalene-Bicarboxylate and Bipyridine Ligands. Inorg. Chem. 2016, 55, 6496–6503. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.R.; Zhu, Q.Y.; Huo, L.B.; Shi, Z.; Bian, G.Q.; Dai, J. Tetrathiafulvalene-tetracarboxylate: An intriguing building block with versatility in coordination structures and redox properties. Inorg. Chem. 2010, 49, 7372–7381. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.D.; Huo, P.; Shao, M.Y.; Yin, J.X.; Shen, W.C.; Zhu, Q.Y.; Dai, J. A new type of charge-transfer salts based on tetrathiafulvalene-tetracarboxylate coordination polymers and methyl viologen. Inorg. Chem. 2014, 53, 3480–3487. [Google Scholar] [CrossRef]
- Jiang, M.; Weng, Y.G.; Zhou, Z.Y.; Ge, C.Y.; Zhu, Q.Y.; Dai, J. Cobalt Metal-Organic Frameworks Incorporating Redox-Active Tetrathiafulvalene Ligand: Structures and Effect of LLCT within the MOF on Photoelectrochemical Properties. Inorg. Chem. 2020, 59, 10727–10735. [Google Scholar] [CrossRef]
- Zhang, S.; Panda, D.K.; Yadav, A.; Zhou, W.; Saha, S. Effects of intervalence charge transfer interaction between pi-stacked mixed valent tetrathiafulvalene ligands on the electrical conductivity of 3D metal-organic frameworks. Chem. Sci. 2021, 12, 13379–13391. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Li, Y.G.; Wen, H.R.; Liu, S.J.; Peng, Y.; Liu, C.M. A family of lanthanide metal-organic frameworks based on a redox-active tetrathiafulvalene-dicarboxylate ligand showing slow relaxation of magnetisation and electronic conductivity. Dalton Trans. 2021, 50, 14714–14723. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.F.; Chan, B.; Faust, T.B.; D’Alessandro, D.M. Controlling charge separation in a novel donor–acceptor metal–organic framework via redox modulation. Chem. Sci. 2014, 5, 4724–4728. [Google Scholar] [CrossRef] [Green Version]
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dinca, M. High charge mobility in a tetrathiafulvalene-based microporous metal-organic framework. J. Am. Chem. Soc. 2012, 134, 12932–12935. [Google Scholar] [CrossRef] [Green Version]
- Park, S.S.; Hontz, E.R.; Sun, L.; Hendon, C.H.; Walsh, A.; van Voorhis, T.; Dinca, M. Cation-dependent intrinsic electrical conductivity in isostructural tetrathiafulvalene-based microporous metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 1774–1777. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Lv, Z.-P.; Leong, C.F.; Zhao, Y.; D’Alessandro, D.M.; Zuo, J.-L. Crystal Structures, Gas Adsorption, and Electrochemical Properties of Electroactive Coordination Polymers Based on the Tetrathiafulvalene-Tetrabenzoate Ligand. Cryst. Growth Des. 2015, 15, 1861–1870. [Google Scholar] [CrossRef]
- Souto, M.; Santiago-Portillo, A.; Palomino, M.; Vitorica-Yrezabal, I.J.; Vieira, B.J.C.; Waerenborgh, J.C.; Valencia, S.; Navalon, S.; Rey, F.; Garcia, H.; et al. A highly stable and hierarchical tetrathiafulvalene-based metal-organic framework with improved performance as a solid catalyst. Chem. Sci. 2018, 9, 2413–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, M.; Romero, J.; Calbo, J.; Vitorica-Yrezabal, I.J.; Zafra, J.L.; Casado, J.; Orti, E.; Walsh, A.; Minguez Espallargas, G. Breathing-Dependent Redox Activity in a Tetrathiafulvalene-Based Metal-Organic Framework. J. Am. Chem. Soc. 2018, 140, 10562–10569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S.; Hendon, C.H.; Fielding, A.J.; Walsh, A.; O’Keeffe, M.; Dinca, M. The Organic Secondary Building Unit: Strong Intermolecular pi Interactions Define Topology in MIT-25, a Mesoporous MOF with Proton-Replete Channels. J. Am. Chem. Soc. 2017, 139, 3619–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S.; Rieth, A.J.; Hendon, C.H.; Dinca, M. Selective Vapor Pressure Dependent Proton Transport in a Metal-Organic Framework with Two Distinct Hydrophilic Pores. J. Am. Chem. Soc. 2018, 140, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Xie, L.S.; Kolobov, N.; Shkurenko, A.; Qureshi, M.; Tchalala, M.R.; Park, S.S.; Bavykina, A.; Eddaoudi, M.; Dincă, M.; et al. Toward New 2D Zirconium-Based Metal–Organic Frameworks: Synthesis, Structures, and Electronic Properties. Chem. Mater. 2019, 32, 97–104. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Wang, T.; Lollar, C.T.; Zuo, J.L.; Zhang, J.; Zhou, H.C. Zirconium metal-organic frameworks incorporating tetrathiafulvalene linkers: Robust and redox-active matrices for in situ confinement of metal nanoparticles. Chem. Sci. 2020, 11, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.S.; Dinca, M. Novel Topology in Semiconducting Tetrathiafulvalene Lanthanide Metal-Organic Frameworks. Isr. J. Chem. 2018, 58, 1119–1122. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Li, J.; Wang, H.Y.; Ge, J.Y.; Drake, H.F.; Leong, C.F.; Yu, F.; D’Alessandro, D.M.; Kurmoo, M.; et al. Rare-Earth Metal Tetrathiafulvalene Carboxylate Frameworks as Redox-Switchable Single-Molecule Magnets. Chem. Eur. J. 2021, 27, 622–627. [Google Scholar] [CrossRef]
- Xie, L.S.; Alexandrov, E.V.; Skorupskii, G.; Proserpio, D.M.; Dinca, M. Diverse pi-pi stacking motifs modulate electrical conductivity in tetrathiafulvalene-based metal-organic frameworks. Chem. Sci. 2019, 10, 8558–8565. [Google Scholar] [CrossRef] [Green Version]
- Castells-Gil, J.; Manas-Valero, S.; Vitorica-Yrezabal, I.J.; Ananias, D.; Rocha, J.; Santiago, R.; Bromley, S.T.; Baldovi, J.J.; Coronado, E.; Souto, M.; et al. Electronic, Structural and Functional Versatility in Tetrathiafulvalene-Lanthanide Metal-Organic Frameworks. Chem. Eur. J. 2019, 25, 12636–12643. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; He, W.; Li, X.-M.; Sun, L.; Wang, H.-Y.; Lan, Y.-Q.; Ding, M.; Zuo, J.-L. High Electrical Conductivity in a 2D MOF with Intrinsic Superprotonic Conduction and Interfacial Pseudo-capacitance. Matter 2020, 2, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Xu, N.; Murase, R.; Yang, Z.M.; D’Alessandro, D.M.; Zuo, J.L.; Zhu, J. Persistent Radical Tetrathiafulvalene-Based 2D Metal-Organic Frameworks and Their Application in Efficient Photothermal Conversion. Angew. Chem. Int. Ed. 2021, 60, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cai, P.; Yan, T.; Yang, Z.M.; Yuan, S.; Zuo, J.L.; Zhou, H.C. Enhancing the photothermal conversion of tetrathiafulvalene-based MOFs by redox doping and plasmon resonance. Chem. Sci. 2022, 13, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hu, T.H.; Murase, R.; Wang, H.Y.; D’Alessandro, D.M.; Kurmoo, M.; Zuo, J.L. Redox Activities of Metal-Organic Frameworks Incorporating Rare-Earth Metal Chains and Tetrathiafulvalene Linkers. Inorg. Chem. 2019, 58, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.F.; Wang, C.-H.; Ling, C.D.; D’Alessandro, D.M. A spectroscopic and electrochemical investigation of a tetrathiafulvalene series of metal–organic frameworks. Polyhedron 2018, 154, 334–342. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Kumazawa, K.; Fujita, M. Room-temperature and solution-state observation of the mixed-valence cation radical dimer of tetrathiafulvalene, [(TTF)2]+*, within a self-assembled cage. J. Am. Chem. Soc. 2005, 127, 13456–13457. [Google Scholar] [CrossRef]
- Lyskawa, J.; Salle, M.; Balandier, J.Y.; le Derf, F.; Levillain, E.; Allain, M.; Viel, P.; Palacin, S. Monitoring the formation of TTF dimers by Na+ complexation. Chem. Commun. 2006, 21, 2233–2235. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Bosch, M. Lanthanide Metal-Organic Frameworks; Springer-Verlag: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Jung, J.; le Natur, F.; Cador, O.; Pointillart, F.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Guizouarn, T.; le Guennic, B.; Bernot, K. Experimental and theoretical evidence that electrostatics governs easy-axis orientation in Dy(III)-based molecular chains. Chem. Commun. 2014, 50, 13346–13348. [Google Scholar] [CrossRef]
- Goura, J.; Guillaume, R.; Rivière, E.; Chandrasekhar, V. Hexanuclear, Heterometallic, Ni3Ln3 Complexes Possessing O-Capped Homo- and Heterometallic Structural Subunits: SMM Behavior of the Dysprosium Analogue. Inorg. Chem. 2014, 53, 7815–7823. [Google Scholar] [CrossRef]
- Program, M. Mercury Program: Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge U.K. Available online: http://www.ccdc.cam.ac.uk/ (accessed on 8 February 2020).
- Görbitz, C.H. What is the best crystal size for collection of X-ray data? Refinement of the structure of glycyl-L-serine based on data from a very large crystal. Acta Cryst. B 1999, 55, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64 Pt 1, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C Struct. Chem. 2015, 71 Pt 1, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Tb-m-TTFTB | Er-m-TTFTB | Gd-m-TTFTB | |
|---|---|---|---|
| CCDC number | 1,914,385 | 1,914,387 | 1,914,384 |
| Empirical formula | C61H53N3O20S6Tb2 | C61H53N3O20S6Er2 | C61H53N3O20S6Gd2 |
| Formula weight | 1658.32 | 1674.99 | 1654.97 |
| Temperature (K) | 153 | 153 | 153 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Space group | P-1 (No. 2) | P-1 (No. 2) | P-1 (No. 2) |
| a (Å) | 15.5977 (10) | 15.371 (16) | 15.5779 (7) |
| b (Å) | 15.696 (1) | 15.15 (3) | 15.7382 (7) |
| c (Å) | 16.6914 (11) | 16.441 (18) | 16.7290 (7) |
| α (°) | 106.077 (1) | 105.29 (4) | 106.761 (1) |
| β (°) | 114.426 (1) | 115.493 (15) | 114.410 (1) |
| γ (°) | 104.542 (1) | 104.25 (3) | 104.065 (2) |
| Volume (Å3) | 3248.0 (4) | 3036 (8) | 3253.0 (3) |
| Z | 2 | 2 | 2 |
| d (g·cm−3) | 1.491 | 1.613 | 1.485 |
| F(000) | 1432 | 1444 | 1428 |
| Absorption coefficient, μ/mm−1 | 2.411 | 3.013 | 2.272 |
| Reflections total | 29,069 | 28,223 | 29,492 |
| Reflections independent | 14,332 | 13,867 | 15,158 |
| θ-range (°) | 1.5–27.3 | 1.5–27.9 | 2.1–27.7 |
| Rint | 0.029 | 0.027 | 0.025 |
| R1, (a) wR2 (I ≥ 2σ(I)) (b) | 0.0327/0.1031 | 0.0445/0.1375 | 0.0295/0.0775 |
| R1, wR2 (all data) | 0.0424/0.1314 | 0.0546/0.1614 | 0.0353/0.0805 |
| GOF | 1.05 | 1.10 | 1.06 |
| Largest diff. peak and hole (eÅ−3) | 1.66/−1.12 | 2.70/−1.71 | 2.49/−1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Yang, Z.-M.; Zhou, X.-C.; Zhang, G.; Su, J. Redox-Active Metal-Organic Frameworks with Three-Dimensional Lattice Containing the m-Tetrathiafulvalene-Tetrabenzoate. Molecules 2022, 27, 4052. https://doi.org/10.3390/molecules27134052
Huang H, Yang Z-M, Zhou X-C, Zhang G, Su J. Redox-Active Metal-Organic Frameworks with Three-Dimensional Lattice Containing the m-Tetrathiafulvalene-Tetrabenzoate. Molecules. 2022; 27(13):4052. https://doi.org/10.3390/molecules27134052
Chicago/Turabian StyleHuang, Hongrui, Zhi-Mei Yang, Xiao-Cheng Zhou, Gen Zhang, and Jian Su. 2022. "Redox-Active Metal-Organic Frameworks with Three-Dimensional Lattice Containing the m-Tetrathiafulvalene-Tetrabenzoate" Molecules 27, no. 13: 4052. https://doi.org/10.3390/molecules27134052
APA StyleHuang, H., Yang, Z. -M., Zhou, X. -C., Zhang, G., & Su, J. (2022). Redox-Active Metal-Organic Frameworks with Three-Dimensional Lattice Containing the m-Tetrathiafulvalene-Tetrabenzoate. Molecules, 27(13), 4052. https://doi.org/10.3390/molecules27134052