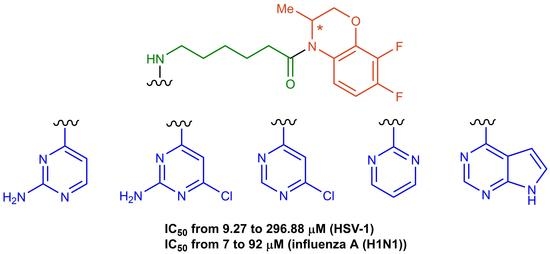
Synthesis of Pyrimidine Conjugates with 4-(6-Amino-hexanoyl)-7,8-difluoro-3,4-dihydro-3-methyl-2H-[1,4]benzoxazine and Evaluation of Their Antiviral Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiviral Evaluation
2.2.1. Anti-Herpesvirus Activity
2.2.2. Anti-Influenza Activity
3. Materials and Methods
3.1. Chemistry General Section
3.2. General Procedure for the Synthesis of the Target Compounds 5a–e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, R.; Chouhan, A. An overview of biological importance of pyrimidines. World J. Pharm. Pharm. Sci. 2014, 3, 574–597. [Google Scholar]
- Roopan, S.M.; Sompalle, R. Synthetic chemistry of pyrimidines and fused pyrimidines: A review. Synth. Commun. 2016, 46, 645–672. [Google Scholar] [CrossRef]
- Kumar, S.; Narasimhan, B. Therapeutic potential of heterocyclic pyrimidine scaffolds. Chem. Cent. J. 2018, 12, 38. [Google Scholar] [CrossRef]
- Kumar, S.; Deep, A.; Narasimhan, B. A Review on Synthesis, Anticancer and Antiviral Potentials of Pyrimidine Derivatives. Curr. Bioact. Compd. 2019, 15, 289–303. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N. Development of new antituberculosis drugs among of 1,3-and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. 2019, 68, 2172–2189. [Google Scholar] [CrossRef]
- Sagma, E.G.; Lakshmanan, B. A review on therapeutic potential of heterocyclic pyrimidine derivatives as potent antiviral agents. Asian J. Pharm. Clin. Res. 2020, 13, 30–34. [Google Scholar]
- Becan, L.; Wojcicka, A. Synthesis, anti-hepatitis B and C virus activity and antitumor screening of novel thiazolo[4,5-d]-pyrimidine derivatives. Acta Pol. Pharm. 2016, 73, 107–114. [Google Scholar]
- Gasparyan, S.P.; Alexanyan, M.V.; Arutyunyan, G.K.; Kocharov, S.L.; Martirosyan, A.H.; Tamazyan, R.A.; Ayvazyan, A.G.; Panosyan, H.A.; Danagulyan, G.G. Synthesis of new derivatives of 5-(3,4-dihydro-2H-pyrrol-5-yl)-pyrimidine. Russ. J. Org. Chem. 2016, 52, 1646–1653. [Google Scholar] [CrossRef]
- Akula, H.K.; Kokatla, H.; Andrei, G.; Snoeck, R.; Schols, D.; Balzarini, J.; Yang, L.; Lakshman, M.K. Facile functionalization at the C4 position of pyrimidine nucleosides via amide group activation with (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP) and biological evaluations of the products. Org. Biomol. Chem. 2017, 15, 1130–1139. [Google Scholar] [CrossRef] [Green Version]
- Slepukhin, P.A.; Voinkov, E.K.; Ulomskiy, E.N.; Savateev, K.V.; Kopchuk, D.S.; Zyryanov, G.V.; Fedotov, V.V.; Charushin, V.N.; Chupakhin, O.N.; Rusinov, V.L. Synthesis and X-ray structural studies of 5-methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4-triazolo[1,5-a]pyrimidine L-arginine and piperidine salts. Chem. Heterocycl. Compd. 2019, 55, 989–992. [Google Scholar] [CrossRef]
- Solomyannyi, R.; Slivchuk, S.; Smee, D.; Choi, J.-a.; Rusanov, E.; Zhirnov, V.; Brovarets, V. In vitro Activity of the Novel Pyrimidines and Their Condensed Derivatives Against Poliovirus. Curr. Bioact. Compd. 2019, 15, 582–591. [Google Scholar] [CrossRef]
- Tao, L.; Li, Y.; Guo, X.; Dong, L.; Liu, L.; Wang, Q.; Yu, X.; Song, C.; Chang, J. Synthesis and anti-CVB3 activity of 4-amino acid derivative substituted pyrimidine nucleoside analogues. Bioorg. Med. Chem. Lett. 2020, 30, 1267704. [Google Scholar] [CrossRef]
- Abu-Zaied, M.A.; Hammad, S.F.; Halaweish, F.T.; Elgemeie, G.H. Sofosbuvir Thio-analogues: Synthesis and Antiviral Evaluation of the First Novel Pyridine- and Pyrimidine-Based Thioglycoside Phosphoramidates. ACS Omega 2020, 5, 14645–14655. [Google Scholar] [CrossRef]
- Abu-Zaied, M.A.; Mahmoud, N.M.; Elgemeie, G.H. Toward Developing Therapies against Corona Virus: Synthesis and Anti-Avian Influenza Virus Activity of Novel Cytosine Thioglycoside Analogues. ACS Omega 2020, 5, 20042–20050. [Google Scholar] [CrossRef]
- Hilmy, K.; Tag, M.; Aish, E.; Elsafty, M.; Attia, H. Synthesis and Biological Evaluation of Pyrrolo[2,3-d]pyrimidine Derivatives as a Novel Class of Antimicrobial and Antiviral Agents. Russ. J. Org. Chem. 2021, 57, 430–439. [Google Scholar]
- Feng, D.; Zuo, X.; Jing, L.; Chen, C.-H.; Olotu, F.A.; Lin, H.; Soliman, M.; De Clercq, E.; Pannecouque, C.; Lee, K.-H.; et al. Design, synthesis, and evaluation of “dual-site”-binding diarylpyrimidines targeting both NNIBP and the NNRTI adjacent site of the HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2021, 211, 113063. [Google Scholar] [CrossRef]
- Drenichev, M.S.; Oslovsky, V.E.; Sun, L.; Tijsma, A.; Kurochkin, N.N.; Tararov, V.I.; Chizhov, A.O.; Neyts, J.; Pannecouque, C.; Leyssen, P.; et al. Modification of the length and structure of the linker of N6-benzyladenosine modulates its selective antiviral activity against enterovirus 71. Eur. J. Med. Chem. 2016, 111, 84–94. [Google Scholar]
- Sala, M.; Kogler, M.; Plackova, P.; Mejdrova, I.; Hrebabecky, H.; Prochazkova, E.; Strunin, D.; Lee, G.; Birkus, G.; Weber, J.; et al. Purine analogs as phosphatidylinositol 4-kinase IIIβ inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2706–2712. [Google Scholar] [CrossRef]
- Venkatesham, A.; Saudi, M.; Kaptein, S.; Neyts, J.; Rozenski, J.; Froeyen, M.; Van Aerschot, A. Aminopurine and aminoquinazoline scaffolds for development of potential dengue virus inhibitors. Eur. J. Med. Chem. 2017, 126, 101–109. [Google Scholar] [CrossRef]
- Tichý, M.; Smoleń, S.; Tloušt’ová, E.; Pohl, R.; Oždian, T.; Hejtmánková, K.; Lišková, B.; Gurská, S.; Džubák, P.; Hajdúch, M.; et al. Synthesis and Cytostatic and Antiviral Profiling of Thieno-Fused 7-Deazapurine Ribonucleosides. J. Med. Chem. 2017, 60, 2411–2424. [Google Scholar] [CrossRef] [Green Version]
- Pomeisl, K.; Pohl, R.; Snoeck, R.; Andrei, G.; Krečmerová, M. Utilization of 1,3-Dioxolanes in the Synthesis of α-branched Alkyl and Aryl 9-[2-(Phosphonomethoxy)ethyl]purines and Study of the Influence of α-branched Substitution for Potential Biological Activity. Nucleosides Nucleotides Nucleic Acids 2019, 38, 119–156. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Musiyak, V.V.; Chulakov, E.N.; Levit, G.L.; Krasnov, V.P. Synthesis of purine and 2-aminopurine conjugates bearing the fragments of heterocyclic amines at position 6. Chem. Heterocycl. Compd. 2015, 51, 738–744. [Google Scholar] [CrossRef]
- Krasnov, V.P.; Gruzdev, D.A.; Chulakov, E.N.; Vigorov, A.Y.; Musiyak, V.V.; Matveeva, T.V.; Tumashov, A.A.; Levit, G.L.; Charushin, V.N. Synthesis of novel purin-6-yl conjugates with heterocyclic amines linked via 6-aminohexanoyl fragment. Mendeleev Commun. 2015, 25, 412–414. [Google Scholar] [CrossRef]
- Eletskaya, B.Z.; Konstantinova, I.D.; Paramonov, A.S.; Esipov, R.S.; Gruzdev, D.A.; Vigorov, A.Y.; Levit, G.L.; Miroshnikov, A.I.; Krasnov, V.P.; Charushin, V.N. Chemoenzymatic arabinosylation of 2-aminopurines bearing the chiral fragment of 7,8-difluoro-3-methyl-3,4-dihydro-2H-[1,4]benzoxazines. Mendeleev Commun. 2016, 26, 6–8. [Google Scholar] [CrossRef]
- Tumashov, A.A.; Gruzdev, D.A.; Vigorov, A.Y.; Musiyak, V.V.; Chulakov, E.N.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Analysis of racemic conjugates of purine with heterocyclic amines by chiral high-performance liquid chromatography. Russ. Chem. Bull. 2018, 67, 1704–1709. [Google Scholar] [CrossRef]
- Eletskaya, B.Z.; Gruzdev, D.A.; Krasnov, V.P.; Levit, G.L.; Kostromina, M.A.; Paramonov, A.S.; Kayushin, A.L.; Muzyka, I.S.; Muravyova, T.I.; Esipov, R.S.; et al. Enzymatic synthesis of novel purine nucleosides bearing a chiral benzoxazine fragment. Chem. Biol. Drug Design. 2019, 93, 605–616. [Google Scholar] [CrossRef]
- Krasnov, V.P.; Musiyak, V.V.; Vozdvizhenskaya, O.A.; Galegov, G.A.; Andronova, V.L.; Gruzdev, D.A.; Chulakov, E.N.; Vigorov, A.Y.; Ezhikova, M.A.; Kodess, M.I.; et al. N-[omega-(Purin-6-yl)aminoalkanoyl] Derivatives of Chiral Heterocyclic Amines as Promising Anti-Herpesvirus Agents. Eur. J. Org. Chem. 2019, 4811–4821. [Google Scholar] [CrossRef]
- Krasnov, V.P.; Levit, G.L.; Musiyak, V.V.; Gruzdev, D.A.; Charushin, V.N. Fragment-based approach to novel bioactive purine derivatives. Pure Appl. Chem. 2020, 92, 1277–1295. [Google Scholar] [CrossRef]
- Andronova, V.L.; Galegov, G.A.; Musiyak, V.V.; Vozdvizhenskaya, O.A.; Levit, G.L.; Krasnov, V.P. Antiviral effect of novel purine conjugate LAS-131 against Herpes simplex virus type 1 (Herpesviridae: Alphaherpesvirinae: Simplexvirus: Human alphaherpesvirus 1) in vitro. Voprosy Virusol. 2021, 65, 373–380. [Google Scholar] [CrossRef]
- Vozdvizhenskaya, O.A.; Andronova, V.L.; Galegov, G.A.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Synthesis and antiherpetic activity of novel purine conjugates with 7,8-difluoro-3-methyl-3,4-dihydro-2H-1,4-benzoxazine. Chem. Heterocycl. Compd. 2021, 57, 490–497. [Google Scholar] [CrossRef]
- Krasnov, V.P.; Zarubaev, V.V.; Gruzdev, D.A.; Vozdvizhenskaya, O.A.; Vakarov, S.A.; Musiyak, V.V.; Chulakov, E.N.; Volobueva, A.S.; Sinegubova, E.O.; Ezhikova, M.A.; et al. Novel purine–N-heterocycle conjugates: Synthesis and anti-influenza activity. Chem. Heterocycl. Compd. 2021, 57, 498–504. [Google Scholar] [CrossRef]
- Brown, D.J. The Pyrimidines. In The Chemistry of Heterocyclic Compounds; Brown, D.J., Mason, S.F., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1962; Volume 16. [Google Scholar]
- van der Westhuyzen, C.W.; Rousseau, A.L.; Parkinson, C.J. Effect of substituent structure on pyrimidine electrophilic substitution. Tetrahedron 2007, 63, 5394–5405. [Google Scholar] [CrossRef]
- Machníková, R.; Janovská, L.; Brulíkov, L. Solid-phase synthetic approach towards new pyrimidines as potential antibacterial agents. J. Mol. Struct. 2020, 1200, 127101. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kogon, I.C.; Minin, R.; Overberger, C.G. 2-Chloropyrimidine. Org. Synth. 1955, 35, 34–35. [Google Scholar]
- Armarego, W.L.F. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Burlington, MA, USA, 2017. [Google Scholar]



| Compound | CC50 ± SD (µM) | HSV-1/L2 | HSV-1/L2/R | ||
|---|---|---|---|---|---|
| IC50 (µM) | SI | IC50 (µM) | SI | ||
| (S)-1 | 293.48 ± 15.55 | 4.6 | 64 | 4.6 | 64 1 |
| (RS)-5a | 150.56 ± 8.36 | 18.56 | 8.1 | 18.56 | 8 |
| (S)-5a | 34.63 ± 0.88 | 9.27 | 3.7 | 9.27 | <4 |
| (RS)-5b | 274.57 ± 4.30 | 73.38 | 3.7 | 73.38 | <4 |
| (RS)-5c | >304.25 | 148.44 | >2.0 | 152.12 | 2 |
| (RS)-5d | 504.77 ± 17.72 | 296.88 | 1.7 | 296.88 | <2 |
| (RS)-5e | 17.58 ± 0.85 | 18.56 | <1 | 18.56 | <1 |
| (S)-5e | 468.97 ± 10.83 | 74.22 | 6.3 | 75.22 | 6 |
| Acyclovir | >444 | 1.73 | >256 | >444 | 1 |
| Foscarnet | >416.61 | 104.15 | >4 | 104.15 | >4 |
| Ribavirin | >4098 | 1025 | >4 | 1025 | >4 |
| Compound | CC50 ± SD (µM) | IC50 (µM) | SI |
|---|---|---|---|
| (S)-1 | 24 ± 1 | >10 | >2 1 |
| (RS)-5a | 11 ± 1 | 8 ± 1 | >1 |
| (S)-5a | 23 ± 1.2 | 7 ± 1 | >3 |
| (RS)-5b | 705 ± 42 | 92 ± 11 | <8 |
| (RS)-5c | 9 ± 1 | >8 | 1 |
| (RS)-5d | 257 ± 12 | >88 | 3 |
| (RS)-5e | 86 ± 4 | >80 | 1 |
| (S)-5e | 47 ± 3 | >26 | <2 |
| Oseltamivir carboxylate | >200 | 0.3 | >667 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnov, V.P.; Musiyak, V.V.; Levit, G.L.; Gruzdev, D.A.; Andronova, V.L.; Galegov, G.A.; Orshanskaya, I.R.; Sinegubova, E.O.; Zarubaev, V.V.; Charushin, V.N. Synthesis of Pyrimidine Conjugates with 4-(6-Amino-hexanoyl)-7,8-difluoro-3,4-dihydro-3-methyl-2H-[1,4]benzoxazine and Evaluation of Their Antiviral Activity. Molecules 2022, 27, 4236. https://doi.org/10.3390/molecules27134236
Krasnov VP, Musiyak VV, Levit GL, Gruzdev DA, Andronova VL, Galegov GA, Orshanskaya IR, Sinegubova EO, Zarubaev VV, Charushin VN. Synthesis of Pyrimidine Conjugates with 4-(6-Amino-hexanoyl)-7,8-difluoro-3,4-dihydro-3-methyl-2H-[1,4]benzoxazine and Evaluation of Their Antiviral Activity. Molecules. 2022; 27(13):4236. https://doi.org/10.3390/molecules27134236
Chicago/Turabian StyleKrasnov, Victor P., Vera V. Musiyak, Galina L. Levit, Dmitry A. Gruzdev, Valeriya L. Andronova, Georgii A. Galegov, Iana R. Orshanskaya, Ekaterina O. Sinegubova, Vladimir V. Zarubaev, and Valery N. Charushin. 2022. "Synthesis of Pyrimidine Conjugates with 4-(6-Amino-hexanoyl)-7,8-difluoro-3,4-dihydro-3-methyl-2H-[1,4]benzoxazine and Evaluation of Their Antiviral Activity" Molecules 27, no. 13: 4236. https://doi.org/10.3390/molecules27134236
APA StyleKrasnov, V. P., Musiyak, V. V., Levit, G. L., Gruzdev, D. A., Andronova, V. L., Galegov, G. A., Orshanskaya, I. R., Sinegubova, E. O., Zarubaev, V. V., & Charushin, V. N. (2022). Synthesis of Pyrimidine Conjugates with 4-(6-Amino-hexanoyl)-7,8-difluoro-3,4-dihydro-3-methyl-2H-[1,4]benzoxazine and Evaluation of Their Antiviral Activity. Molecules, 27(13), 4236. https://doi.org/10.3390/molecules27134236