Evaluation of Phytochemistry and Pharmacological Properties of Alnus nitida
Abstract
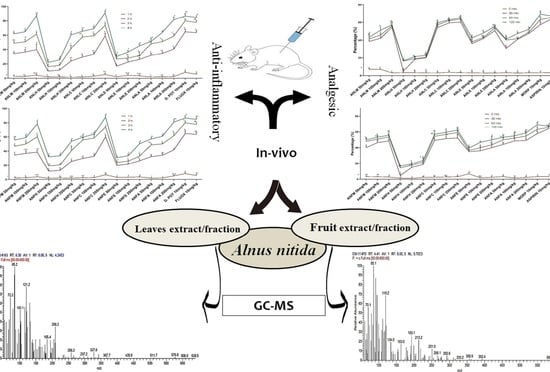
:1. Introduction
2. Results
2.1. Acute Toxicity Studies
2.2. Assessment of Anti-Inflammatory Activity
2.2.1. Carrageenan-Induced Paw Edema
2.2.2. Freunds’ Complete Adjuvant Induced Arthritis
2.2.3. Histamine Induced Paw Edema Method
2.2.4. Xylene-Induced Ear Edema
2.3. Analgesic Activity of A. nitida
2.3.1. Hotplate Analgesic Test
2.3.2. Acetic Acid-Induced Writhing Test
2.4. GC-MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Sampling
4.2. Extract Preparation
4.3. Animals
4.4. Acute Toxicity Study
4.5. Assessment of Anti-Inflammatory Activity
4.5.1. Carrageenan-Induced Hind Paw Edema Test
4.5.2. Freunds’ Complete Adjuvant Induced Arthritis
4.5.3. Histamine Induced Paw Edema Method
4.5.4. Xylene-Induced Ear Edema Method
4.6. Assessment of Analgesic Activity
4.6.1. Hotplate Analgesic Test
4.6.2. Acetic Acid-Induced Writhing Test
4.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gou, K.-J.; Zeng, R.; Dong, Y.; Hu, Q.-Q.; Hu, H.-W.-Y.; Maffucci, K.G.; Dou, Q.-L.; Yang, Q.-B.; Qin, X.-H.; Qu, Y. Anti-inflammatory and analgesic effects of Polygonum orientale L. extracts. Front. Pharmacol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M. Aromatherapy & immunity: How the use of essential oil aids immune potentiality: Part 3 immune responses to inflammation and essential oils useful in inhibiting them. Int. J. Aromather. 2001, 11, 220–224. [Google Scholar]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; De la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Richardson, J.D.; Vasko, M.R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine induces microglia activation and the release of proinflammatory mediators in rat brain via H1R or H4R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Z. Für Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef]
- Tripathi, K. Cardiovascular Drugs. In Essentials of Medical Pharmacology, 5th ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2003; pp. 453–454. [Google Scholar]
- Raffa, R.B. Pharmacology of oral combination analgesics: Rational therapy for pain. J. Clin. Pharm. 2001, 26, 257–264. [Google Scholar] [CrossRef]
- Afilalo, M.; Morlion, B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician 2013, 16, 27–40. [Google Scholar] [CrossRef]
- Thomas, M.C.; Diuretics, A.C.E. Inhibitors and NSAIDs—The triple whammy. Med. J. Aust. 2000, 172, 184–185. [Google Scholar] [CrossRef]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18,820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [Green Version]
- León, J.A.M.; Santisteban, A.G.; Fuentes, D.P.; Puebla, Y.G.; Rodríguez, E.T. In vitro anti-inflammatory activity of aqueous, ethanolic and ethereal extracts of rhizomes, leaves and stems of Anredera vesicaria. J. Anal. Pharm. Res. 2018, 7, 459–461. [Google Scholar]
- Tubon, I.; Zannoni, A.; Bernardini, C.; Salaroli, R.; Bertocchi, M.; Mandrioli, R.; Vinueza, D.; Antognoni, F.; Forni, M. In Vitro Anti-Inflammatory Effect of Salvia sagittata Ethanolic Extract on Primary Cultures of Porcine Aortic Endothelial Cells. Oxidative Med. Cell Longev. 2019, 2019, 6829173. [Google Scholar] [CrossRef]
- Zhang, H.L.; Gan, X.Q.; Fan, Q.F.; Yang, J.J.; Zhang, P.; Hu, H.B.; Song, Q.S. Chemical constituents and anti-inflammatory activities of Maqian (Zanthoxylum myriacanthum var. pubescens) bark extracts. Sci. Rep. 2017, 7, 45805. [Google Scholar] [CrossRef] [Green Version]
- Naz, R.; Ayub, H.; Nawaz, S.; Islam, Z.U.; Yasmin, T.; Bano, A.; Wakeel, A.; Zia, S.; Roberts, T.H. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complement. Altern. Med. 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, S.; Chopra, R. The wealth of India. CSIR India 1948, 1, 8. [Google Scholar]
- Stewart, R.R. Flora of West Pakistan: An Annotated Catalogue of the Vascular Plants of West Pakistan and Kashmir; Fakhri Printing Press: Karachi, Pakistan, 1972; pp. 1–1028. [Google Scholar]
- Lv, H.; She, G. Naturally occurring diarylheptanoids—A supplementary version. Rec. Nat. Prod. 2012, 6, 321–333. [Google Scholar]
- Jin, W.; Cai, X.F.; Na, M.; Lee, J.J.; Bae, K. Diarylheptanoids from Alnus hirsuta inhibit the NF-kB activation and NO and TNF-alpha production. Biol. Pharm. Bull. 2007, 30, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Kim, J.H.; Jeong, D.W.; Ahn, K.H.; Toh, S.H.; Surh, Y.J. Inhibition of cyclooxygenase-2 expression by diarylheptanoids from the bark of Alnus hirsuta var. sibirica. Biol. Pharm. Bull. 2000, 23, 517–518. [Google Scholar] [CrossRef] [Green Version]
- Tung, N.H.; Kwon, H.J.; Kim, J.H.; Ra, J.C.; Ding, Y.; Kim, J.A.; Kim, Y.H. Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg. Med. Chem. Lett. 2010, 20, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, H.J.; Jung, S.Y.; Yook, C.S.; Jin, C.; Lee, Y.S. A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem. Pharm. Bull. 2010, 58, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.N.; Ahmad, V.U.; Zahoor, A.; Ahmed, A.; Khan, S.S.; Khan, A.; Hassan, Z. Two new diarylheptanoids from Alnus nitida. Nat. Prod. Commun. 2010, 5, 1787–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.A.; Shah, N.A.; Ullah, S.; Alam, M.M.; Badshah, H.; Ullah, S.; Mumtaz, A.S. Documenting the indigenous knowledge on medicinal flora from communities residing near Swat River (Suvastu) and in high mountainous areas in Swat-Pakistan. J. Ethnopharmacol. 2016, 182, 67–79. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.R.; Shah, S.A.; Majid, M.; Ismail, H.; Maryam, S.; Batool, R.; Younis, T. Investigations on anti-inflammatory and analgesic activities of Alnus nitida Spach (Endl). stem bark in Sprague Dawley rats. J. Ethnopharmacol. 2017, 198, 407–416. [Google Scholar] [CrossRef]
- Fukawa, K.; Kawano, O.; Hibi, M.; Misaki, N.; Ohba, S.; Hatanaka, Y. A method for evaluating analgesic agents in rats. J. Pharmacol. Methods 1980, 4, 251–259. [Google Scholar]
- Arayne, M.S.; Sultana, N.; Mirza, A.Z.; Zuberi, M.H.; Siddiqui, F.A. In vitro hypoglycemic activity of methanolic extract of some indigenous plants. Pak. J. Pharm. Sci. 2007, 20, 268–273. [Google Scholar]
- Majid, M.; Khan, M.R.; Shah, N.A.; Ul Haq, I.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complement. Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef] [Green Version]
- Younis, T.; Khan, M.R.; Sajid, M. Protective effects of Fraxinus xanthoxyloides (Wall.) leaves against CCl 4 induced hepatic toxicity in rat. BMC Complement. Altern. Med. 2016, 16, 407. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.O.; Sousa, F.B.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.; Sousa, D.P.; Aragao, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fund. Clin. Pharm. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Noguchi, N. Dynamics of antioxidant action of vitamin E. Acc. Chem Res. 2004, 37, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Kavitha Ch, N.; Babu, S.M.; Reddy, V.P.; Latha, A.K. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund’s adjuvant-induced arthritis in albino rats. Inflammation 2012, 35, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Benly, P. Role of histamine in acute inflammation. J. Pharm. Sci. Res. 2015, 7, 373–376. [Google Scholar]
- Yong, Y.K.; Zakaria, Z.A.; Kadir, A.A.; Somchit, M.N.; Ee Cheng Lian, G.; Ahmad, Z. Chemical constituents and antihistamine activity of Bixa orellana leaf extract. BMC Complement. Altern. Med. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharm. Rev. 2001, 53, 597–652. [Google Scholar]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar]
- Carretero, M.E.; Lopez-Perez, J.L.; Abad, M.J.; Bermejo, P.; Tillet, S.; Israel, A.; Noguera, P.B. Preliminary study of the anti-inflammatory activity of hexane extract and fractions from Bursera simaruba (Linneo) Sarg. (Burseraceae) leaves. J. Ethnopharmacol. 2008, 116, 11–15. [Google Scholar] [CrossRef]
- Jananie, R.; Priya, V.; Vijayalakshmi, K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. NY Sci. J. 2011, 4, 16–20. [Google Scholar]
- Yang, L.; Qin, L.H.; Bligh, S.W.; Bashall, A.; Zhang, C.F.; Zhang, M.; Wang, Z.T.; Xu, L.S. A new phenanthrene with a spirolactone from Dendrobium chrysanthum and its anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 3496–3501. [Google Scholar] [CrossRef]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kim, J.H.; Gao, X.; Zhou, J.-L.; Lee, I.; Chung, K.; Chung, J.M. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 2006, 122, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.; de Oliveira, G.A.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [Green Version]
- Al-Qurainy, F.; Khan, S.; Ali, M.A.; Al-Hemaid, F.M.; Tarroum, M.; Ashraf, M. Authentication of Ruta graveolens and its adulterant using internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. Pak. J. Bot. 2011, 43, 1613–1620. [Google Scholar]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latha, R.M.; Geetha, T.; Varalakshmi, P. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen. Pharm. 1998, 31, 601–606. [Google Scholar] [CrossRef]
- Shah, S.M.M. A possible anti-inflammatory mechanism of ethyl acetate extracts of Teucrium stocksianum Bioss. BMC Complement. Altern. Med. 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Núñez Guillén, M.E.; da Silva Emim, J.A.; Souccar, C.; Lapa, A.J. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int. J. Pharm. 1997, 35, 99–104. [Google Scholar] [CrossRef]
- Singh, V.P.; Jain, N.K.; Kulkarni, S.K. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001, 915, 218–226. [Google Scholar] [CrossRef]
- Yahagi, T.; Yakura, N.; Matsuzaki, K.; Kitanaka, S. Inhibitory effect of chemical constituents from Artemisia scoparia Waldst. et Kit. on triglyceride accumulation in 3T3-L1 cells and nitric oxide production in RAW 264.7 cells. J. Nat. Med. 2014, 68, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Saeed, M.; Gilani, A.H.; Muhammad, N.; Haq, I.U.; Ashraf, N.; Rehman, N.U.; Haleemi, A. Antipyretic and anticonvulsant activity of Polygonatum verticillatum: Comparison of rhizomes and aerial parts. Phytother. Res. 2013, 27, 468–471. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kim, J.Y.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005, 71, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.; Anderson, T.; Cavagnino, D.; Patel, A. Comparative analysis of mass spectral matching for confident compound identification using the advanced electron ionization source for GC-MS. Thermoscientific 2018, 10598, 1–7. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]







| S. No. | Compound | Area % | Class | RT | SI | RSI |
|---|---|---|---|---|---|---|
| 1. | Phytol | 1.10 | Diterpene alcohol | 25.17 | 792 | 798 |
| 2. | Lucenin 2 | 1.95 | Ketone | 37.18 | 429 | 437 |
| 3. | Hexadecanoic acid, 2-phenyl-1,3-dioxan-5-yl ester | 0.17 | Ester | 39.08 | 347 | 392 |
| 4. | 5-Phenyl-4-trimethylsilyldibenzophosphole | 15.23 | Phosphol | 32.02 | 726 | 916 |
| 5. | 3Z-3′S-gamma-IRONE | 0.31 | Alkene | 10.80 | 768 | 907 |
| 6. | 2-(Phenylsulfanyl)cyclohexanol | 0.76 | Alcohol | 39.64 | 391 | 681 |
| 7. | Bicyclo [3.2.0]hepta-2,6-diene | 0.16 | Deuterated cyclic Alkane | 37.81 | 386 | 796 |
| 8. | 2-tert-Butyl-4-trifluoromethyl-1-methylimidazole | 0.07 | Amine | 12.96 | 866 | 948 |
| 9. | Rhodopin | 0.11 | Carotenoid | 38.59 | 380 | 387 |
| 10. | Phenanthrene | 0.33 | Arene | 21.73 | 389 | 727 |
| 11. | trans-4,7-Dimethyl-6,7-dihydro-5H-cyclopenta[c]pyridin-5-ol | 0.09 | Alcohol | 10.30 | 503 | 761 |
| 12. | Cyclohexane, 1,1′,1″,1‴-(1,6-hexanediylidene)tetrakis | 0.08 | Alkane | 36.57 | 413 | 427 |
| 13. | 15-Isobutyl-(13àH)-isocopalane | 0.32 | Alkane | 33.09 | 339 | 369 |
| 14. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 0.26 | Terpene Alcohol | 20.77 | 848 | 875 |
| 15. | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 65.38 | Ester | 31.38 | 606 | 754 |
| 16. | 1,3,5-Trimethyl-2-octadecylcyclohexane | 0.11 | Alkane | 33.58 | 419 | 434 |
| 17. | Neophytadiene | 0.89 | Alkene | 19.89 | 867 | 887 |
| 18. | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 0.18 | Ester | 30.79 | 541 | 690 |
| 19. | Ethanone | 0.10 | Ketone | 23.56 | 395 | 433 |
| 20. | Thiopheno[b,b′]dicamphore 1,1-dioxide | 1.68 | Terpenoid | 35.62 | 552 | 862 |
| 21. | 4-Bromobutanoic acid, tridec-2-ynyl ester | 0.06 | Ester | 22.25 | 872 | 569 |
| 22. | Terephthalic acid, dodecyl 2-ethylhexyl ester | 1.14 | Ester | 34.57 | 553 | 641 |
| 23. | 6-Amino-1-[2-(3,4-dimethoxy-phenyl)-ethyl]-1H-pyrimidine-2,4-dione | 0.10 | Acid Amide | 28.98 | 378 | 453 |
| S. No. | Compound | Area % | Class | RT | SI | RSI |
|---|---|---|---|---|---|---|
| 1. | Squalene | 2.38 | Triterpene | 36.15 | 704 | 747 |
| 2. | Pluchidiol | 1.08 | Alcohol | 19.20 | 400 | 565 |
| 3. | Neophytadiene | 1.25 | Alkene | 19.99 | 858 | 881 |
| 4. | trans-8-Ethoxybicyclo [4.3.0]-3-nonene-7-carboxylic acid | 0.07 | Acid | 4.41 | 422 | 425 |
| 5. | 1-[(hexadeuterio)phenyl]naphthalene | 0.93 | Cyclic Alkene | 39.30 | 395 | 745 |
| 6. | 4-(p-Hydroxyphenyl)butyric acid | 0.05 | Acid | 17.37 | 371 | 793 |
| 7. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 0.23 | Terpene Alcohol | 25.27 | 750 | 836 |
| 8. | 2-Chloro-3-formylindol-1-carboxylic acid, 2,2,2-trichloroethyl ester | 0.61 | Ester | 38.39 | 338 | 435 |
| 9. | 5-(2-cyclohexen-1-ylthio)-1-phenyl-1H-tetrazole | 2.30 | Thioether | 6.36 | 363 | 694 |
| 10. | 15-methyltricyclo [6.5.2(13,14).0(7,15)]pentadeca-1,3,5,7,9,11,13-heptene | 0.07 | Terpene | 13.09 | 806 | 862 |
| 11. | Olean-12-ene-3,15,16,21,22,28-hexol | 1.43 | Alcohol | 37.01 | 334 | 350 |
| 12. | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 0.79 | Ester | 34.67 | 560 | 757 |
| 13. | 24,25-Dihydroxycholecalciferol | 0.12 | Tri-Alcohol | 36.71 | 395 | 455 |
| 14. | 3Z-3′S-gamma-IRONE | 0.40 | Ketone | 10.91 | 705 | 829 |
| 15. | tert-Butyl (Z)-3-(trifluoromethyl)-2-decenoate | 0.10 | Derivatives of fatty acids andorganoflurines | 31.07 | 364 | 815 |
| 16. | 1,2-Benzisothiazol-3(2H)-one, 2-(phenylmethyl)-, 1,1-dioxide | 0.07 | Isothiazolinones | 29.10 | 359 | 665 |
| 17. | 1-Cyclohexene-1-acrylic acid,2,6,6-trimethyl-3-oxo-,methyl ester | 0.13 | Ester | 10.40 | 436 | 453 |
| 18. | 4-(3-cyclohexylpropyl)-1,7-heptanediyl]bis | 0.07 | Alkane | 27.04 | 384 | 407 |
| 19. | endo-(4aRS,8RS,8aRS)-8a-(Benzyloxycarbonyl)-1-oxo-2-(p-toluenesulfonyl)-8-(trimethylsiloxy)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline | 1.83 | Benzopyridines | 33.16 | 393 | 797 |
| 20. | Vitamin E | 4.26 | Alcohol | 32.37 | 746 | 934 |
| 21. | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 77.87 | Ester | 31.45 | 502 | 619 |
| 22. | 1-Methyl-4-(9H-xanthen-9-ylidene)piperidine | 0.33 | Ether | 21.83 | 408 | 613 |
| 23. | Decahydronaphtho [2,3-b]furan-2-one, 3-[[(furan-2-ylmethyl)amino]methyl]-4a-hydroxy-5-methoxy-5,8a-dimethyl | 0.69 | Ester | 30.21 | 349 | 432 |
| 24. | 1-[4,4-Dimethyl-6-(2-oxopropyl)-1-oxaspiro [2.3]hex-2-yl]propan-2-one | 0.11 | Di-ketone | 23.66 | 440 | 502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, M.; Khan, M.R.; Ijaz, M.U.; Ismail, H.; Bhatti, M.Z.; Shah, S.A.; Ali, S.; Tareen, M.U.; Alotaibi, S.S.; Albogami, S.M.; et al. Evaluation of Phytochemistry and Pharmacological Properties of Alnus nitida. Molecules 2022, 27, 4582. https://doi.org/10.3390/molecules27144582
Sajid M, Khan MR, Ijaz MU, Ismail H, Bhatti MZ, Shah SA, Ali S, Tareen MU, Alotaibi SS, Albogami SM, et al. Evaluation of Phytochemistry and Pharmacological Properties of Alnus nitida. Molecules. 2022; 27(14):4582. https://doi.org/10.3390/molecules27144582
Chicago/Turabian StyleSajid, Moniba, Muhammad Rashid Khan, Muhammad Umar Ijaz, Hammad Ismail, Muhammad Zeeshan Bhatti, Sayed Afzal Shah, Saima Ali, Muhammad Usman Tareen, Saqer S. Alotaibi, Sarah M. Albogami, and et al. 2022. "Evaluation of Phytochemistry and Pharmacological Properties of Alnus nitida" Molecules 27, no. 14: 4582. https://doi.org/10.3390/molecules27144582
APA StyleSajid, M., Khan, M. R., Ijaz, M. U., Ismail, H., Bhatti, M. Z., Shah, S. A., Ali, S., Tareen, M. U., Alotaibi, S. S., Albogami, S. M., & Batiha, G. E. -S. (2022). Evaluation of Phytochemistry and Pharmacological Properties of Alnus nitida. Molecules, 27(14), 4582. https://doi.org/10.3390/molecules27144582