Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review
Abstract
:1. Introduction
2. Phenolic Compounds
2.1. General Overview
2.2. Phenolic Compounds’ Occurrence in Opuntia sp.
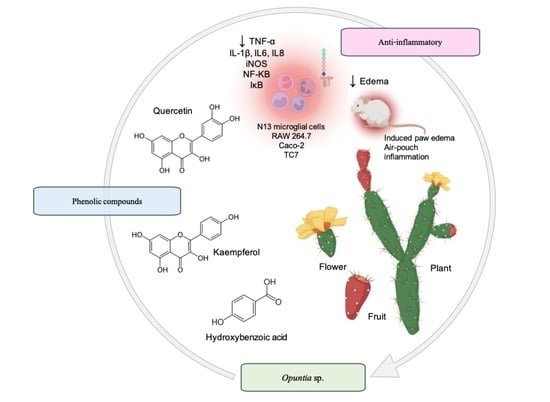
3. Opuntia sp. in Inflammation
3.1. Modulation of Inflammatory Mediators and Enzymes
3.2. Anti-Inflammatory Activity In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sáenz, C. Opuntias as a natural resource. In Agro-Industrial Utilization of Cactus Pear; Sáenz, C., Berger, H., Rodríguez-Félix, A., Galletti, L., García, J.C., Sepúlveda, E., Varnero, M.T., García de Cortázar, V., García, R.C., Arias, E., et al., Eds.; Rural Infrastructure and Agro-Industries Division: Rome, Italy, 2013; pp. 17–21. [Google Scholar]
- El-Mostafa, K.; El-Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El-Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labra, M.; Grassi, F.; Bardini, M.; Imazio, S.; Guiggi, A.; Citterio, S.; Banfi, E.; Sgorbati, S. Genetic Relationships in Opuntia Mill. Genus (Cactaceae) Detected by Molecular Marker. Plant Sci. 2003, 165, 1129–1136. [Google Scholar] [CrossRef]
- Hahm, S.-W.; Park, J.; Oh, S.-Y.; Lee, C.-W.; Park, K.-Y.; Kim, H.; Son, Y.-S. Anticancer Properties of Extracts from Opuntia humifusa against Human Cervical Carcinoma Cells. J. Med. Food 2015, 18, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Callen, E.O. Analysis of the Tehuacan coprolites. In The Prehistory of the Tehuacan Valley; Byers, D.S., Ed.; University of Texas Press: London, UK, 1967; Volume 1, pp. 261–289. [Google Scholar]
- Santos-Díaz, M.S.; Balch, E.P.M.; Ramírez-Malagón, R.; Nuñez-Palenius, H.G.; Ochoa-Alejo, N. Mexican threatened cacti: Current status and strategies for their conservation. In Species Diversity and Extinction; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 1–59. [Google Scholar]
- Kamble, S.M.; Debaje, P.P.; Ranveer, R.C.; Sahoo, A. Nutritional Importance of Cactus: A Review. Trends Biosci. 2017, 10, 7668–7677. [Google Scholar]
- Ochoa, M.J.; Barbera, G. History and economic and agro-ecological importance. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Mondragon, C., Nefzaoui, A., Sáenz, C., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; pp. 1–11. [Google Scholar]
- Ganopoulos, I.; Kalivas, A.; Kavroulakis, N.; Xanthopoulou, A.; Mastrogianni, A.; Koubouris, G.; Madesis, P. Genetic Diversity of Barbary Fig (Opuntia ficus-indica) Collection in Greece with ISSR Molecular Markers. Plant Gene 2015, 2, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Zeghbib, W.; Boudjouan, F.; Bachir-bey, M. Optimization of Phenolic Compounds Recovery and Antioxidant Activity Evaluation from Opuntia ficus indica Using Response Surface Methodology. J. Food Meas. Charact. 2022, 16, 1354–1366. [Google Scholar] [CrossRef]
- Gurrieri, S.; Miceli, L.; Lanza, C.M.; Tomaselli, F.; Bonomo, R.P.; Rizzarelli, E. Chemical Characterization of Sicilian Prickly Pear (Opuntia ficus indica) and Perspectives for the Storage of Its Juice. J. Agric. Food Chem. 2000, 48, 5424–5431. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia ficus-indica Flowers Obtained by Different Extraction Methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological Actions of Opuntia ficus indica: A Review. J. App. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Leem, K.-H.; Kim, M.-G.; Hahm, Y.-T.; Kim, H.K. Hypoglycemic Effect of Opuntia Ficus-Indica Var. Saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/P38 MAPK Pathway. Nutrients 2016, 8, 800. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia ficus indica) at Varied Ages in an Organic Harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slimen, I.B.; Mabrouk, M.; Hanène, C.; Najar, T.; Abderrabba, M. LC-MS Analysis of Phenolic Acids, Flavonoids and Betanin from Spineless Opuntia ficus-indica Fruits. Cell Biol. 2017, 5, 17–28. [Google Scholar] [CrossRef]
- Zenteno-Ramírez, G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Monreal-Montes, M.; García, J.M.; Serratosa, M.P.; Santos, M.Á.V.; Pérez, M.D.O.; Rendón-Huerta, J.A. Juices of Prickly Pear Fruits (Opuntia spp.) As Functional Foods. Ital. J. Food Sci. 2018, 30, 614–627. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; de Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Loredo, M.G.; García-Ochoa, F.; Barragán-Huerta, B.E. Comparative Analysis of Betalain Content in Stenocereus Stellatus Fruits and Other Cactus Fruits Using Principal Component Analysis. Int. J. Food Prop. 2016, 19, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Murevanhema, Y.Y.; Jideani, V.A.; Oguntibeju, O.O. Review on potential of seeds and value-added products of bambara groundnut (Vigna Subterranea): Antioxidant, anti-inflammatory, and anti-oxidative stress. In Bioactive Compounds of Medicinal Plants; Goyal, M.R., Ayeleso, A.O., Eds.; Apple Academic Press: New York, NY, USA, 2018; pp. 102–141. ISBN 978-1-315-14747-5. [Google Scholar]
- De Santiago, E.; Gill, C.I.R.; Carafa, I.; Tuohy, K.M.; de Peña, M.-P.; Cid, C. Digestion and Colonic Fermentation of Raw and Cooked Opuntia ficus-indica Cladodes Impacts Bioaccessibility and Bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef]
- Osuna-Martínez, L.; Reyes Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A Review on Its Antioxidants Properties and Potential Pharmacological Use in Chronic Diseases. Stud. Nat. Prod. Chem. 2014, 2, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-920-1. [Google Scholar]
- Shan, S.; Huang, X.; Shah, M.H.; Abbasi, A.M. Evaluation of Polyphenolics Content and Antioxidant Activity in Edible Wild Fruits. Biomed. Res. Int. 2019, 2019, e1381989. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S. Chapter two—Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural Anti-Inflammatory Agents for Pain Relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–24. ISBN 978-0-470-98855-8. [Google Scholar]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic Compounds and Antioxidant Activity of Kernels and Shells of Mexican Pecan (Carya Illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Chan, S.-O.; Pang, C.P.; Wang, C.C. Pro-Oxidative and Antioxidative Controls and Signaling Modification of Polyphenolic Phytochemicals: Contribution to Health Promotion and Disease Prevention? J. Agric. Food Chem. 2014, 62, 4026–4038. [Google Scholar] [CrossRef] [PubMed]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of Phenolic Compounds from Color and Flavor Problems to Health Benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant extracts as antioxidant additives for food industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018; pp. 87–116. ISBN 978-1-78923-379-7. [Google Scholar]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and Quantification of Phenolic Acids and Flavonols from Eugenia Pyriformis Using Different Solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Nutr. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Ben Amira, A.; Attia, H. Phenolic Profiles, Phytchemicals and Mineral Content of Decoction and Infusion of Opuntia ficus-indica Flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef]
- Ben Lataief, S.; Zourgui, M.-N.; Rahmani, R.; Najjaa, H.; Gharsallah, N.; Zourgui, L. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Bioactive Compounds Extracted from Opuntia dillenii Cladodes. J. Food Meas. Charact. 2021, 15, 782–794. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M. Ultrasound and Microwave Assisted Extraction of Opuntia Fruit Peels Biocompounds: Optimization and Comparison Using RSM-CCD. Molecules 2019, 24, 3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of Micronutrients, Bioaccessibility and Antioxidant Activity of Prickly Pear Cladodes as Functional Ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Ouerghemmi, I.; Ksouri, R.; Wannes, W.A.; Hammrouni, I.; Marzouk, B. HPLC-Determination of Phenolic Composition and Antioxidant Capacity of Cactus Prickly Pears Seeds. Asian J. Chem. 2011, 23, 1006–1010. [Google Scholar]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Angelotti, A.; Bouaziz, F.; Chaabouni, S.E.; Vorobiev, E. Seed Oil Extraction from Red Prickly Pear Using Hexane and Supercritical CO2: Assessment of Phenolic Compound Composition, Antioxidant and Antibacterial Activities. J. Sci. Food Agric. 2017, 97, 613–620. [Google Scholar] [CrossRef]
- López-Palacios, C.; Peña-Valdivia, C.B. Screening of Secondary Metabolites in Cladodes to Further Decode the Domestication Process in the Genus Opuntia (Cactaceae). Planta 2020, 251, 74. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, İ.; Karababa, E. Analytical Evaluation of Phenolic Compounds and Minerals of Opuntia robusta J.C. Wendl. and Opuntia ficus-barbarica A. Berger. Int. J. Food Prop. 2018, 21, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Amrane-Abider, M.; Nerin, C.; Canellas, E.; Benkerrou, F.; Louaileche, H. Modeling and Optimization of Phenolic Compounds Extraction from Prickly Pear (Opuntia ficus-indica) Seeds via Ultrasound-Assisted Technique. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2018, 42, 109–121. [Google Scholar]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic Compound Profile and Biological Activities of Southern African Opuntia ficus-indica Fruit Pulp and Peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Nair, V.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Wounding and UVB Light Synergistically Induce the Biosynthesis of Phenolic Compounds and Ascorbic Acid in Red Prickly Pears (Opuntia ficus-indica Cv. Rojo Vigor). Int. J. Mol. Sci. 2019, 20, 5327. [Google Scholar] [CrossRef] [Green Version]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; del Santos-Díaz, M.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical Composition and Phenolic Compounds Profile of Cladodes from Opuntia spp. Cultivars with Different Domestication Gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the Characterization of Opuntia spp. Juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Lanuzza, F.; Occhiuto, F.; Monforte, M.T.; Tripodo, M.M.; D’Angelo, V.; Galati, E.M. Antioxidant Phytochemicals of Opuntia ficus-indica (L.) Mill. Cladodes with Potential Anti-Spasmodic Activity. Pharmacogn. Mag. 2017, 13, S424–S429. [Google Scholar] [CrossRef] [PubMed]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-Product Recovery of Opuntia spp. Peels: Betalainic and Phenolic Profiles and Bioactive Properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Ouerghemmi, I.; Harbeoui, H.; Aidi Wannes, W.; Bettaieb Rebey, I.; Hammami, M.; Marzouk, B.; Saidani Tounsi, M. Phytochemical Composition and Antioxidant Activity of Tunisian Cactus Pear (Opuntia ficus indica L.) Flower. J. Food Biochem. 2017, 41, 1–10. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; de Peña, M.-P. Digestibility of (Poly)phenols and Antioxidant Activity in Raw and Cooked Cactus Cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; del Rio, D.; Hernández, F. Phytochemical Characterization of Different Prickly Pear (Opuntia ficus-indica (L.) Mill.) Cultivars and Botanical Parts: UHPLC-ESI-MSn Metabolomics Profiles and Their Chemometric Analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and Quantification of Individual Betalain and Phenolic Compounds in Mexican and Spanish Prickly Pear (Opuntia ficus-indica L. Mill) Tissues: A Comparative Study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kita, A.; Miedzianka, J.; Andreu-Coll, L.; Legua, P.; Hernandez, F. Characterization of Bioactive Compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars. Molecules 2020, 25, 5734. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Phytochemical Characterization and Antioxidant Activity of Opuntia microdasys (Lehm.) Pfeiff Flowers in Different Stages of Maturity. J. Funct. Foods. 2014, 9, 27–37. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia spp. as a Novel High Potential by-Product: Phytochemical Characterization and Antioxidant Activity. Ind Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.M.; Sallam, E.M. Utilization of Prickly Pear Peels to Improve Quality of Pan Bread. Arab. J. Nucl. Sci. Appl. 2016, 49, 151–163. [Google Scholar]
- Cruz-Bravo, R.K.; Guzmán-Maldonado, S.H.; Araiza-Herrera, H.A.; Zegbe, J.A. Storage Alters Physicochemical Characteristics, Bioactive Compounds and Antioxidant Capacity of Cactus Pear Fruit. Postharvest Biol. Technol. 2019, 150, 105–111. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var dillenii Fruits and Products of Their Industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil Composition and Characterisation of Phenolic Compounds of Opuntia ficus-indica Seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Ammar, I.; Ben Salem, M.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-Inflammatory Activity and Phenolic Composition of Prickly Pear (Opuntia ficus-indica) Flowers. Ind. Crops Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and Inflammatory Disease: Synthetic and Natural Antioxidants with Anti-Inflammatory Activity. Comb. Chem. High Throughput Screen 2006, 9, 425–442. [Google Scholar] [CrossRef]
- Linus, L.O.; Hanson, C.; Alolga, R.N.; Zhou, W.; Qi, L. Targeting the Key Factors of Inflammation in Cancer: Plant Intervention. Int. J. Clin. Exp. Med. 2017, 10, 15834–15865. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.L.; Blikslager, A. The Future of Anti-inflammatory Therapy. Vet. Clin. N. Am. 2001, 17, 245–262. [Google Scholar]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-Inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-κB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Chen, R.; Jiang, C.; Chen, L.; Cheng, Z. Correlation of LOX-5 and COX-2 Expression with Inflammatory Pathology and Clinical Features of Adenomyosis. Mol. Med. Rep. 2019, 19, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Sig. Transduct. Target Ther. 2021, 6, 1–30. [Google Scholar] [CrossRef]
- Cuzzo, B.; Lappin, S.L. Physiology, leukotrienes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ahmed, M.S.; El Tanbouly, N.D.; Islam, W.T.; Sleem, A.A.; El Senousy, A.S. Antiinflammatory Flavonoids from Opuntia dillenii (Ker-Gawl) Haw. Flowers Growing in Egypt. Phytother. Res. 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, S.C.; Kim, T.W.; Kim, K.S.; Song, J.-C.; Kim, S.K.; Lee, H.M.; Sung, H.J.; Park, H.J.; Song, Y.B.; et al. Radical Scavenging and Anti-Inflammatory Activity of Extracts from Opuntia humifusa Raf. J. Pharm. Pharmacol. 2006, 58, 113–119. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Sheth, N.R.; Suhagia, B.N. Analgesic and Anti-Inflammatory Action of Opuntia elatior Mill Fruits. J. Ayurveda Integr. Med. 2015, 6, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Benattia, F.K.; Arrar, Z.; Khabbal, Y. Evaluation of the Anti-Inflammatory Activity of the Seeds Extracts of Prickly Pear (Opuntia ficus-indica L.). Der Pharma Chem. 2017, 9, 14–17. [Google Scholar]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical Composition, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Opuntia stricta Cladodes. PLoS ONE 2019, 14, e0209682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, A.; Nunes, S.L.; Poejo, J.; Mecha, E.; Serra, A.T.; Madeira, P.J.A.; Bronze, M.R.; Duarte, C.M.M. Antioxidant and Anti-Inflammatory Activity of a Flavonoid-Rich Concentrate Recovered from Opuntia ficus-indica Juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; López-Pacheco, F.; Alvarez, M.M.; Serna-Saldívar, S.O. In Vivo Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica (L.) Mill Cladodes. Ind. Crops Prod. 2015, 76, 803–808. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory Potential of Prickly Pears and Their Isolated Bioactives against Digestive Enzymes Linked to Type 2 Diabetes and Inflammatory Response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- McCook, J.P.; Dorogi, P.L.; Vasily, D.B.; Cefalo, D.R. In Vitro Inhibition of Hyaluronidase by Sodium Copper Chlorophyllin Complex and Chlorophyllin Analogs. Clin. Cosmet. Investig. Dermatol. 2015, 8, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of Hyaluronidase Activity by Vitis rotundifolia. (Muscadine) Berry Seeds and Skins. Pharm. Biol. 2007, 45, 667–673. [Google Scholar] [CrossRef]
- González-Peña, D.; Colina-Coca, C.; Char, C.D.; Cano, M.P.; de Ancos, B.; Sánchez-Moreno, C. Hyaluronidase Inhibiting Activity and Radical Scavenging Potential of Flavonols in Processed Onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of Anti-Inflammatory and Antioxidant Activities of Prickly Pear Fruits by High Hydrostatic Pressure: A Chemical and Microstructural Approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Chaalal, M.; Gavilán, E.; Louaileche, H.; Ruano, D.; Parrado, J.; Castaño, A. Anti-Inflammatory Activity of Phenolic Extracts from Different Parts of Prickly Pear on Lipopolysaccharide-Stimulated N13 Microglial Cells. Int. J. Phytomed. 2016, 7, 411–419. [Google Scholar]
- Yeo, J.Y.; Hwang, K.W.; Park, S.-Y. Anti-Inflammatory Effect of Neo-Lignan Isoamericanin A via Suppression of NF-κB in Liposaccharide-Stimulated RAW 264.7 Cells. Trop. J. Pharm. Res. 2020, 19, 1857–1862. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; Angelis, M.D.; Silano, M.; Gobbetti, M.; Cagno, R.D. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef] [Green Version]




| Phenolic Compounds | Plant Tissue | Concentration (µg/g) | Opuntia Species | References |
|---|---|---|---|---|
| Flavonoids | ||||
| Flavones | ||||
| Apigenin | Seeds Cladodes Flowers | NS 0.19–0.65 NS | O. stricta O. ficus-indica O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [38,42,43,44] |
| Luteolin | Pulp Peel | NS NS | O. ficus-barbarica O. robusta | [45] |
| Flavonols | ||||
| Myricetin | Seeds Pulp Peel Cladodes | 198.19–428.14 NS NS 8.52 | O. ficus-indica O. ficus-barbarica O. robusta | [45,46,47] |
| Rutin | Seeds Pulp Peel Cladodes | 8.00–100.00 9.70–12.50 65.70–103.40 2.11–4.95 | O. ficus-indica O. ficus-barbarica O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [42,44,45,48,49] |
| Quercetin and derivates | Seeds Pulp Peel Cladodes Flowers | 4.37–18.77 84.20–599.20 715.70–1316.20 8.97–75.13 NS | O. ficus-indica O. ficus-barbarica O. robusta O. engelmannii O. streptacantha O. hyptiacantha O. megacantha O. albicarpa | [12,38,42,44,45,47,49,50,51,52,53,54,55,56,57,58] |
| Kaempferol and derivates | Pulp Peel Cladodes Flowers | 207.10–529.10 52.90–675.50 72.97–241.68 321.00–708.00 | O. ficus-indica O. engelmannii O. streptacantha O. hyptiacantha O. megacantha O. albicarpa O. microdasys | [38,47,48,49,50,52,53,54,55,57,59] |
| Isorhamnetin and derivates | Seeds Pulp Peel Cladodes Flowers | 67.14–288.58 29.30–58.40 1484.70–2213.70 1250.00–4140.00 NS | O. ficus-indica O. microdasys O. stricta O. streptacantha O. hyptiacantha O. megacantha O. albicarpa | [12,19,38,44,46,47,48,50,51,52,53,54,55,56,57,59,60] |
| Flavanones | ||||
| Naringenin | Pulp Peel | 210.00 20.00–180.00 | O. ficus-indica O. ficus-barbarica O. robusta | [45,56] |
| Flavanols | ||||
| Catechin | Seeds Pulp Peel Cladodes Flowers | NS 14.44–27.89 NS 180.00 NS | O. stricta O. ficus-indica O. megacantha O. streptacantha O. robusta | [17,38,43,49,52,54,61] |
| Epicatechin | Seeds Pulp Peel | NS 19.16–90.81 NS | O. ficus-indica O. albicarpa O. megacantha O. streptacantha O. robusta | [17,42,61] |
| Gallocatechin | Seeds Pulp Peel | NS 116.60–178.20 120.40–334.70 | O. stricta O. ficus-indica | [43,49] |
| Epigallocatechin | Seeds | NS | O. stricta O. ficus-indica | [42,43] |
| Anthocyanidins | ||||
| Pelargonidin | Seeds Cladodes | NS 187.97 | O. stricta O. ficus-indica | [43,47] |
| Cyanidin | Seeds Cladodes | NS 1058.57 | O. stricta O. ficus-indica | [43,47] |
| Delphinidin | Cladodes | 2.81 | O. ficus-indica | [47] |
| Petunidin | Cladodes | 186.55 | O. ficus-indica | [47] |
| Malvidin | Cladodes | 4.31 | O. ficus-indica | [47] |
| Phenolic Acids | ||||
| Gallic acid and derivates | Seeds Pulp Peel Cladodes Flowers | NS 32.60–81.20 NS 20.53–38.96 NS | O. ficus-indica O. stricta O. ficus-barbarica O. robusta O. albicarpa O. megacantha O. streptacantha O. hyptiacantha | [17,42,43,44,45,49,54,61,62,63] |
| Ferulic acid and derivates | Seeds Pulp Peel Cladodes Flowers | 96.33–1366.24 80.00 150.00–390.00 130.00–370.00 291.00–786.00 | O. ficus-indica O. stricta O. ficus-barbarica O. microdasys O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [12,43,44,45,46,48,49,51,52,55,56,59,61,64] |
| Caffeic acid and derivates | Seeds Pulp Peels Cladodes Flowers | NS NS NS NS 255.00–469.00 | O. ficus-indica O. ficus-barbarica O. robusta O. microdasys O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [12,38,42,44,45,48,49,51,54,59,61] |
| Sinapic acid | Seeds Pulp Peel Cladodes | NS 100.00–4100.00 820.00–2350.00 40.00–750.00 | O. stricta O. ficus-indica | [43,49,56] |
| p-Coumaric acid | Seeds Pulp Peel Cladodes Flowers | NS NS NS 20.91 65.00–178.00 | O. ficus-indica O. ficus-barbarica O. robusta O. microdasys O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [42,44,45,48,49,52,59] |
| Hydroxycinnamic acid | Cladodes | 8.45–1248.24 | O. ficus-indica O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [44,47] |
| Chlorogenic acid | Seeds Cladodes | 885.31–1148.41 5.00–26.49 | O. ficus-indica O. streptacantha O. hyptiacantha O. megacantha O. albicarpa | [42,44,46,50,52] |
| Ellagic acid | Seeds Pulp Peel | 73.74–74.38 25.00–73.20 NS | O. ficus-indica O. megacantha O. streptacantha O. robusta O. ficus-indica | [17,46,61] |
| Vanillic acid | Seeds Pulp Peel Cladodes Flowers | NS NS NS 0.11–24.30 NS | O. stricta O. ficus-barbarica O. robusta O. ficus-indica O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [43,44,45,49,54,61] |
| Syringic acid | Seeds Pulp Peel Cladodes Flowers | NS 13.60–66.50 NS 2.34–13.99 NS | O. ficus-indica O. robusta O. albicarpa O. megacantha O. streptacantha O. hyptiacantha O. stricta | [12,17,42,43,44,45,54,61] |
| Protocatechuic acid | Seeds Pulp Peel | 4.57–22.36 NS NS | O. ficus-indica O. ficus-barbarica O. robusta O. stricta | [45,46,49,58,61,62,63] |
| Hydroxybenzoic acid | Pulp Peel Cladodes | 200.90–816.80 964.00–1718.20 114.01 | O. ficus-indica O. hyptiacantha O. streptacantha O. megacantha O. albicarpa | [44,47,49,57,62] |
| Piscidic acid | Seeds Pulp Peel Cladodes | NS NS NS NS | O. ficus-indica O. stricta | [48,51,53,55,57,58,63] |
| Eucomic acid | Seeds Pulp Peel Cladodes | NS NS NS NS | O. ficus-indica O. streptacantha O. hyptiacantha O. megacantha O. albicarpa O. stricta | [48,50,51,53,55,58,63] |
| Gentisic acid | Pulp Peel | NS NS | O. ficus-barbarica O. robusta | [45] |
| Rosmarinic acid | Peel Flowers | NS NS | O. ficus-indica | [49,54] |
| Catechol | Seeds Pulp Peel | NS NS NS | O. stricta O. ficus-barbarica O. robusta O. ficus-indica | [43,45,61] |
| Other Phenolics | ||||
| Phloretin Psoralen Pinoresinol | Seeds | NS | O. stricta | [43] |
| Species (Tissue) | Compounds | Dose | Model | Mechanism of Action | Ref. | |
|---|---|---|---|---|---|---|
| In Vitro Studies |
Opuntia ficus-indica (seeds, pulp, fruits) | Phenolic compounds from crude extracts | 10 mg/mL |
LPS-stimulated murine N13 microglial cells |
| [90] |
|
Opuntia humifusa (cladodes) |
Phenolic compound from crude extracts |
0.05/0.1 mg/mL |
LPS-stimulated Macrophages’ RAW 264.7 |
| [78] | |
|
Opuntia humifusa (seeds) | Isoamericanin A |
1.0–4.0 µg/mL |
LPS-stimulated Macrophages’ RAW 264.7 |
| [91] | |
|
Opuntia ficus-indica (cladodes) | Crude extract | 10 mg/mL |
Human intestinal Caco-2/TC7 cells |
| [92] | |
|
Opuntia ficus-indica (fruits) |
Polyphenols from crude extract | 0.05 mg/mL |
Human colon carcinoma Caco-2 cells |
| [82] | |
| In Vivo Studies |
Opuntia dillenii (stems, flowers, fruits) | Kaempferol 3-O-α-arabinoside Isorhamnetin-3-O-β-d-glucopyranoside Isorhamnetin-3-O-β-d-rutinoside |
50 mg/kg BW |
Carrageenan-induced paw edema in Albino rats |
| [77] |
|
Opuntia ficus-indica (flowers) | Phenolic compounds from crude extract |
400 mg/kg BW |
Carrageenan-induced paw edema in Wistar rats |
| [65] | |
|
Opuntia ficus-indica (cladodes) |
Isorhamnetin-3-O- glucosyl-rhamnoside Isorhamnetin-3-O- glucosyl-rhamnosyl-rhamnoside |
5 mg/kg BW |
Carrageenan-induced air-pouch inflammation in Wistar rats |
| [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. https://doi.org/10.3390/molecules27154763
Zeghbib W, Boudjouan F, Vasconcelos V, Lopes G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules. 2022; 27(15):4763. https://doi.org/10.3390/molecules27154763
Chicago/Turabian StyleZeghbib, Walid, Fares Boudjouan, Vitor Vasconcelos, and Graciliana Lopes. 2022. "Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review" Molecules 27, no. 15: 4763. https://doi.org/10.3390/molecules27154763
APA StyleZeghbib, W., Boudjouan, F., Vasconcelos, V., & Lopes, G. (2022). Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules, 27(15), 4763. https://doi.org/10.3390/molecules27154763