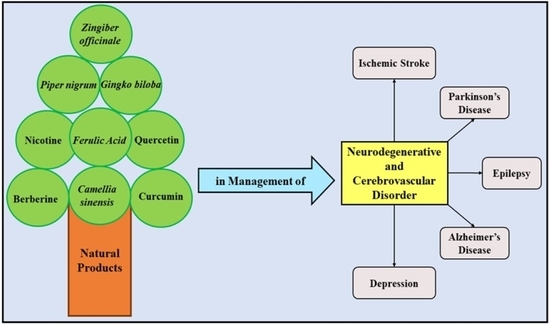
Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders
Abstract
:1. Introduction
2. Potential Therapeutic Molecules of Natural Products against Neurodegenerative and Cerebrovascular Diseases
3. Natural Products and Their Protective Role in the Management of Alzheimer’s Disease
3.1. Activation and Targeting of Cholinergic Neurotransmission
3.2. Stimulation of Inhibitory Activity of BACE-1
3.3. Inhibition of α-Synuclein
| Name of the Plant | Target of Action | Remarks | Ref. |
|---|---|---|---|
| Piper nigrum (Black Pepper) | AchE, CRP, NF-ĸB | Increased levels of Ach in brain Decreased levels of serum inflammatory cytokines in brain Prevents neuroinflammation and promotes cholinergic neurotransmission | [35] |
| Foeniculum vulgare (Fennel) | AchE | Decreases the activation of AchE enzyme in brain Prevention of amnesia | [36] |
| Ocimum sanctum (Tulsi) | AchE | Decreases the activation of AchE enzyme in cortex, medulla, cerebellum and mid-regions of the brain Prevention of cognitive behaviour | [37] |
| Lavandula angustifolia Mill. (Lavender) | AChE, Matrix metalloproteinase, ROS | Decreases the activation of AchE enzyme in brain Increased antioxidant activity Decreased production of reactive oxygen species and nitric oxide Overall improvement in cognitive behaviour | [38] |
| Olea europaea (Olive) | MDA, NO, COX-2 | Decreases the production of reactive oxygen species, malondialdehyde and nitric oxide Minimizes oxidative stress | [39] |
| Camellia sinensis (Green tea) | AChE, COX | Improved cholinergic functions | [40] |
| Ajmalicine | BACE-1 | Inhibits the activity of BACE-1 enzyme by binding to its catalytic site | [41] |
| Berberine | BACE-1 | Inhibits the activity of BACE-1 enzyme non-competitively by binding to methylenedioxy group at the D ring of the enzyme | [42] |
| Gallic acid | BACE-1 | Improved learning and memory Amelioration of cerebral amyloidosis | [43] |
| Epigallocatechin-3-gallate | α-synuclein | Inhibits the aggregation of α-synuclein protein and prevents further accumulation of Aβ-plaque | [44] |
| Hypericum afrum | MAO A and MAO B | Decreased production of amyloid plaques | [45] |
| Cytisusvillosus | MAO A and MAO B | Enhancement of dopaminergic neurotransmission | [45] |
| Curcuma longa | MAO A and MAO B | Strong MAO inhibition and thus prevention of further deterioration of the disease | [46] |
4. Natural Products and Their Protective Role in Management of Parkinson’s Disease
4.1. Mucuna pruriens
4.2. Nicotine
4.3. Phytic Acid
4.4. Pepper
4.5. Ginger
5. Natural Products and Their Protective Role in the Management of Depression
5.1. Curcumin
5.2. Apigenin
5.3. Amentoflavone
5.4. Chlorogenic Acid
5.5. Ellagic Acid
5.6. Ferulic Acid
6. Natural Products and Their Protective Role in Management of Ischemic Stroke
7. Challenges, Future Prospects and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| AchE | Acetylcholinesterase |
| AICD | APP intracellular domain |
| AIF | apoptosis inducing factor |
| APP | amyloid precursor protein |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid beta |
| BACE-1 | β-secretase |
| BDNF | Brain derived neurotrophic factors |
| BuChE | Butyrylcholinesterase |
| CAG | cytosine-adenine-guanine |
| cAMP | cyclic adenosine monophosphate |
| ChAT | O-acetyltransferase |
| COX | cyclooxygenase |
| CREB | cAMP response element-binding protein |
| CRP | C reaction protein |
| DNA | deoxy ribonucleic acids |
| EGCG | Epigallocatechin-3-gallate |
| GABA | gamma aminobutyric acid |
| HDAC | Histone deacetylase |
| IL-1β | interleukin 1 β |
| MAO A | Monoamine oxidase A |
| MAO B | Monoamine oxidase B |
| MAO | Monoamine oxidase |
| MDA | malondialdehyde |
| MMP | Matrix metalloproteinase |
| mRNA | messenger ribonucleic acid |
| nAchR | nicotinic acetylcholine receptors |
| NF-ĸB | Nuclear factor kappa B |
| NO | Nitric oxide |
| PARP 1 | poly ADP-ribose polymerase-1 |
| PI3K | phosphatidylinositol 3-kinase |
| p-JNK | c-Jun-N-terminal kinase |
| PKA | protein kinase A |
| ROS | reactive oxygen species |
| sAPPβ | soluble amyloid precursor proteins |
| TNF-α | Tumor necrosis factor-α |
References
- Makkar, R.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M. Targeting Insulin-Like Growth Factor-I in Management of Neurological Disorders. Neurotox. Res. 2022, 40, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.-H.; Dumbrava, D.-A. Dietary Habits, Lifestyle Factors and Neurodegenerative Diseases. Neural Regen. Res. 2020, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an Ethical Challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef]
- Pallag, A.; Bungau, S.G.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids, and Chlorophylls in Equisetum Arvense T. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Troncoso-Escudero, P.; Sepulveda, D.; Pérez-Arancibia, R.; Parra, A.V.; Arcos, J.; Grunenwald, F.; Vidal, R.L. On the Right Track to Treat Movement Disorders: Promising Therapeutic Approaches for Parkinson’s and Huntington’s Disease. Front. Aging Neurosci. 2020, 12, 571185. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the Evolution and Pathophysiology of Alzheimer Disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Pidgeon, C.; Rickards, H. The Pathophysiology and Pharmacological Treatment of Huntington Disease. Behav. Neurol. 2013, 26, 245–253. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Stoothoff, W.H.; de Calignon, A.; Jones, P.B.; Hyman, B.T. Tau Pathophysiology in Neurodegeneration: A Tangled Issue. Trends Neurosci. 2009, 32, 150–159. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C. Current Trends in Neurodegeneration: Cross Talks between Oxidative Stress, Cell Death, and Inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, Oxidative Stress and Neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Fu, L.-M.; Li, J.-T. A Systematic Review of Single Chinese Herbs for Alzheimer’s Disease Treatment. Evid.-Based Complement. Altern. Med. 2011, 2011, 640284. [Google Scholar] [CrossRef] [Green Version]
- Geun Kim, H.; Sook Oh, M. Herbal Medicines for the Prevention and Treatment of Alzheimer’s Disease. Curr. Pharm. Des. 2012, 18, 57–75. [Google Scholar] [CrossRef]
- Dos Santos-Neto, L.L.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; de Souza, G.A. The Use of Herbal Medicine in Alzheimer’s Disease—A Systematic Review. Evid.-Based Complement. Altern. Med. 2006, 3, 441–445. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Zhang, X.; Wang, Y. Herbal Therapy: A New Pathway for the Treatment of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2010, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Akhondzadeh, S.; Abbasi, S.H. Herbal Medicine in the Treatment of Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2006, 21, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sanka, N.; Santhipriya, N.; Nadendla, R.R. An Updated Review on Anti-Alzheimer’s Herbal Drugs. J. Drug Deliv. Ther. 2018, 8, 360–372. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, T.; Mołdoch, J.; Kowalska, I.; Szponar, J.; Oniszczuk, A. Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review. Int. J. Mol. Sci. 2022, 23, 1212. [Google Scholar] [CrossRef]
- Pang, X.-C.; Kang, D.; Fang, J.-S.; Zhao, Y.; Xu, L.-J.; Lian, W.W.; Liu, A.-L.; Du, G.-H. Network Pharmacology-Based Analysis of Chinese Herbal Naodesheng Formula for Application to Alzheimer’s Disease. Chin. J. Nat. Med. 2018, 16, 53–62. [Google Scholar]
- Chen, G.; Xie, Y.; Yang, Y.; Zhou, D.; Hao, Y.; Liu, Y.; Cheng, M.; Hou, Y.; Li, N. Natural Therapeutic Agents for Neurodegenerative Diseases from the Shells of Xanthoceras sorbifolium. Bioorg. Chem. 2020, 101, 104038. [Google Scholar] [CrossRef]
- Ali, S.K.; Hamed, A.R.; Soltan, M.M.; Hegazy, U.M.; Elgorashi, E.E.; El-Garf, I.A.; Hussein, A.A. In-Vitro Evaluation of Selected Egyptian Traditional Herbal Medicines for Treatment of Alzheimer Disease. BMC Complement. Altern. Med. 2013, 13, 121. [Google Scholar] [CrossRef] [Green Version]
- Howes, M.-J.R.; Fang, R.; Houghton, P.J. Effect of Chinese Herbal Medicine on Alzheimer’s Disease. Int. Rev. Neurobiol. 2017, 135, 29–56. [Google Scholar]
- Ferreira, J.P.S.; Albuquerque, H.M.T.; Cardoso, S.M.; Silva, A.M.S.; Silva, V.L.M. Dual-Target Compounds for Alzheimer’s Disease: Natural and Synthetic AChE and BACE-1 Dual-Inhibitors and Their Structure-Activity Relationship (SAR). Eur. J. Med. Chem. 2021, 221, 113492. [Google Scholar] [CrossRef]
- Abdul, B.A.A.; Vidhyavathi, R.; Magesh, J.; Vijayakumar, M.; Mustafa, M.M.; Marikar, F.M.M.T. Synthesis and Development of BACE 1 Inhibitor for Alzheimer’s Diseases from Medicinal Plants. Annu. Res. Rev. Biol. 2018, 28, 1–18. [Google Scholar] [CrossRef]
- Wang, X.; Perumalsamy, H.; Kwon, H.W.; Na, Y.-E.; Ahn, Y.-J. Effects and Possible Mechanisms of Action of Acacetin on the Behavior and Eye Morphology of Drosophila Models of Alzheimer’s Disease. Sci. Rep. 2015, 5, 16127. [Google Scholar] [CrossRef]
- Sheela, D.; Rohan, R. A Pathophysiological and Pharmacological Review on Alzheimer’s Disease: A Current Need. Biomed. Pharmacol. J. 2021, 14, 75–81. [Google Scholar] [CrossRef]
- Ardah, M.T.; Ghanem, S.S.; Abdulla, S.A.; Lv, G.; Emara, M.M.; Paleologou, K.E.; Vaikath, N.N.; Lu, J.-H.; Li, M.; Vekrellis, K. Inhibition of Alpha-Synuclein Seeded Fibril Formation and Toxicity by Herbal Medicinal Extracts. BMC Complement. Med. Ther. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ardah, M.T.; Durairajan, S.S.K.; Liu, L.; Xie, L.; Fong, W.D.; Hasan, M.Y.; Huang, J.; El-Agnaf, O.M.A.; Li, M. Baicalein Inhibits Formation of A-Synuclein Oligomers within Living Cells and Prevents Aβ Peptide Fibrillation and Oligomerisation. ChemBioChem 2011, 12, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, K.M.; Hasan, M.Y.; Al-Hayani, A.; El-Agnaf, O.M.A. The Role of α-Synuclein in Neurodegenerative Diseases: From Molecular Pathways in Disease to Therapeutic Approaches. Curr. Alzheimer Res. 2013, 10, 559–568. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Salem, A.M.; Sabry, G.M.; Husein, A.A.; Kotob, S.E. Possible Therapeutic Uses of Salvia triloba and Piper nigrum in Alzheimer’s Disease–Induced Rats. J. Med. Food 2013, 16, 437–446. [Google Scholar] [CrossRef]
- Pala, A.; Serdar, O.; MişeYonar, S.; Yonar, M.E. Ameliorative Effect of Fennel (Foeniculum vulgare) Essential Oil on Chlorpyrifos Toxicity in Cyprinus carpio. Environ. Sci. Pollut. Res. 2021, 28, 890–897. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Mani, V.; Ashok Dundapa, T.; Watanabe, K.; Konishi, T. Ocimum sanctum Linn. Leaf Extracts Inhibit Acetylcholinesterase and Improve Cognition in Rats with Experimentally Induced Dementia. J. Med. Food 2011, 14, 912–919. [Google Scholar] [CrossRef]
- Oskouie, A.A.; Yekta, R.F.; Tavirani, M.R.; Kashani, M.S.; Goshadrou, F. Lavandula Angustifolia Effects on Rat Models of Alzheimer’s Disease through the Investigation of Serum Metabolic Features Using NMR Metabolomics. Avicenna J. Med. Biotechnol. 2018, 10, 83. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid.-Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.E.; Taşkin, T. Camellia sinensis Leaves Hydroalcoholic Extract Improves the Alzheimer’s Disease-Like Alterations Induced by Type 2 Diabetes in Rats. Clin. Exp. Health Sci. 2020, 10, 93–103. [Google Scholar]
- Kashyap, P.; Kalaiselvan, V.; Kumar, R.; Kumar, S. Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands towards Factors Implicated in Alzheimer’s Disease. Molecules 2020, 25, 1609. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Wang, C.; Yang, W. Role of Berberine in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic Acid Is a Dual α/β-Secretase Modulator That Reverses Cognitive Impairment and Remediates Pathology in Alzheimer Mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential Neuroprotective Properties of Epigallocatechin-3-Gallate (EGCG). Nutr. J. 2015, 15, 60. [Google Scholar] [CrossRef] [Green Version]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D. Inhibition of Human Monoamine Oxidase A and B by Flavonoids Isolated from Two Algerian Medicinal Plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the Potential Clinical Value of Curcumin in the Prevention and Diagnosis of Alzheimer’s Disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Liu, S.; Lu, F. Recent Advances in Herbal Medicines Treating Parkinson’s Disease. Fitoterapia 2013, 84, 273–285. [Google Scholar] [CrossRef]
- Mythri, R.B.; Harish, G.; Bharath, M.M. Therapeutic Potential of Natural Products in Parkinson’s Disease. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 181–200. [Google Scholar] [CrossRef]
- Sarrafchi, A.; Bahmani, M.; Shirzad, H.; Rafieian-Kopaei, M. Oxidative Stress and Parkinson’s Disease: New Hopes in Treatment with Herbal Antioxidants. Curr. Pharm. Des. 2016, 22, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-H.; Cho, K.-H.; Jung, W.-S.; Lee, M.S. Herbal Medicines for Parkinson’s Disease: A Systematic Review of Randomized Controlled Trials. PLoS ONE 2012, 7, e35695. [Google Scholar] [CrossRef] [Green Version]
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The Magic Velvet Bean of Mucuna pruriens. J. Tradit. Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef]
- Yadav, S.K.; Rai, S.N.; Singh, S.P. Mucuna pruriens Reduces Inducible Nitric Oxide Synthase Expression in Parkinsonian Mice Model. J. Chem. Neuroanat. 2017, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Franklin, G.; Longstreth, W.; Swanson, P.; Checkoway, H. Nicotine, Nightshades and Parkinson’s Disease. Aust. J. Herb. Med. 2013, 25, 155–156. [Google Scholar]
- Girdhar, S.; Girdhar, A.; Verma, S.K.; Lather, V.; Pandita, D. Plant Derived Alkaloids in Major Neurodegenerative Diseases: From Animal Models to Clinical Trials. J. Ayurvedic Herb. Med. 2015, 1, 91–100. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Mei, C.; Ning, X.; Pang, J.; Gu, L.; Wu, L. Phytic Acid Exerts Protective Effects in Cerebral Ischemia-Reperfusion Injury by Activating the Anti-Oxidative Protein Sestrin2. Biosci. Biotechnol. Biochem. 2020, 84, 1401–1408. [Google Scholar] [CrossRef]
- Handa, V.; Sharma, D.; Kaur, A.; Arya, S.K. Biotechnological Applications of Microbial Phytase and Phytic Acid in Food and Feed Industries. Biocatal. Agric. Biotechnol. 2020, 25, 101600. [Google Scholar] [CrossRef]
- Dhanalakshmi, C.; Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M.; Khan, M.A.S. Spices and Parkinson’s Disease. In Food and Parkinson’s Disease; Nova Science Publishing, Inc.: New York, NY, USA, 2016; p. 59. [Google Scholar]
- Milenković, A.N.; Stanojević, L.P. Black Pepper: Chemical Composition and Biological Activities. Adv. Technol. 2021, 10, 40–50. [Google Scholar] [CrossRef]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Prabhala, H.K.; Pai, C.; Prabhala, R.H. Anti-Inflammatory Natural Foods. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases: Bioactive Food in Chronic Disease States; Elsevier: Amsterdam, The Netherlands, 2012; p. 279. [Google Scholar]
- Liu, L.; Liu, C.; Wang, Y.; Wang, P.; Li, Y.; Li, B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr. Neuropharmacol. 2015, 13, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal Medicine for Depression, Anxiety and Insomnia: A Review of Psychopharmacology and Clinical Evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal Medicine for Depression and Anxiety: A Systematic Review with Assessment of Potential Psycho-Oncologic Relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural Polyphenols in the Management of Major Depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef]
- Andrade, C. A Critical Examination of Studies on Curcumin for Depression. J. Clin. Psychiatry 2014, 75, 3929. [Google Scholar] [CrossRef] [Green Version]
- Nazemi, H.; Mirzaei, M.; Jafari, E. Antidepressant Activity of Curcumin by Monoamine Oxidase–A Inhibition. J. Adv. Chem. B 2019, 1, 3–9. [Google Scholar]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef]
- Jalali, A.; Firouzabadi, N.; Zarshenas, M.M. Pharmacogenetic-Based Management of Depression: Role of Traditional Persian Medicine. Phyther. Res. 2021, 35, 5031–5052. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s Wort (Hypericum perforatum): A Review of the Current Pharmacological, Toxicological, and Clinical Literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef]
- Ishola, I.O.; Chatterjee, M.; Tota, S.; Tadigopulla, N.; Adeyemi, O.O.; Palit, G.; Shukla, R. Antidepressant and Anxiolytic Effects of Amentoflavone Isolated from Cnestis ferruginea in Mice. Pharmacol. Biochem. Behav. 2012, 103, 322–331. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B.; Han, P.-L.; Lee, J.-K.; Suh, H.-W. Antidepressant-like Effect of Chlorogenic Acid Isolated from Artemisia capillaris Thunb. Anim. Cells Syst. 2010, 14, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Mehan, S.; Khanna, D.; Kalra, S. Polyphenol Ellagic Acid–Targeting to Brain: A Hidden Treasure. Int. J. Neurol. Res. 2015, 1, 141–152. [Google Scholar]
- Rafieirad, M.; Nezhad, Z.Z.; Allahbakhshi, E. Neuroprotective Effects of Oral Ellagic Acid on Locomotor Activity and Anxiety-Induced by Ischemia/Hypoperfusion in Rat. Adv. Environ. Biol. 2014, 8, 83–89. [Google Scholar]
- Khatri, D.K.; Juvekar, A.R. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Pharmacogn. Mag. 2016, 12, S116. [Google Scholar] [PubMed] [Green Version]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Grilli, M.; Pittaluga, A.M.; Boggia, R. Ellagic Acid a Multi-Target Bioactive Compound for Drug Discovery in CNS? A Narrative Review. Eur. J. Med. Chem. 2019, 183, 111724. [Google Scholar] [CrossRef]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Wang, Y.; Xie, Y.; Qiu, X.; Ren, P.; Gao, L.; Zhou, H.; Zhang, H.; Qiao, M. Ferulic Acid-Induced Anti-Depression and Prokinetics Similar to Chaihu–Shugan–San via Polypharmacology. Brain Res. Bull. 2011, 86, 222–228. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Shao, T.; Ruan, L.; Wang, L.; Sun, J.; Li, J.; Zhu, X.; O’Donnell, J.M.; Pan, J. Ferulic Acid Increases Pain Threshold and Ameliorates Depression-like Behaviors in Reserpine-Treated Mice: Behavioral and Neurobiological Analyses. Metab. Brain Dis. 2013, 28, 571–583. [Google Scholar] [CrossRef]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural Medicine in Neuroprotection for Ischemic Stroke: Challenges and Prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef]
- Cetin, N.; Dasdelen, D.; Mogulkoc, R.; Menevse, E.; Baltaci, A.K. Role of Exogenous Putrescine in the Status of Energy, DNA Damage, Inflammation, and Spermidine/Spermine-n (1)-Acetyltransferase in Brain Ischemia-Reperfusion in Rats. Iran. J. Basic Med. Sci. 2022, 25, 597–603. [Google Scholar]
- Gaire, B.P. Herbal Medicine in Ischemic Stroke: Challenges and Prospective. Chin. J. Integr. Med. 2018, 24, 243–246. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.; Wan, Q. Therapeutic Targets of Neuroprotection and Neurorestoration in Ischemic Stroke: Applications for Natural Compounds from Medicinal Herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Qiao, H.; Li, Y.; Xia, M.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Xie, Y. Research Progress of Natural Products for the Treatment of Ischemic Stroke. J. Integr. Neurosci. 2022, 21, 14. [Google Scholar] [CrossRef]
- Zhang, B.; Saatman, K.E.; Chen, L. Therapeutic Potential of Natural Compounds from Chinese Medicine in Acute and Subacute Phases of Ischemic Stroke. Neural Regen. Res. 2020, 15, 416. [Google Scholar]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L. Totarol Prevents Neuronal Injury in Vitro and Ameliorates Brain Ischemic Stroke: Potential Roles of Akt Activation and HO-1 Induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Ren, J.-X.; Li, C.; Yan, X.-L.; Qu, Y.; Yang, Y.; Guo, Z.-N. Crosstalk between Oxidative Stress and Ferroptosis/Oxytosis in Ischemic Stroke: Possible Targets and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, L.; Yuan, X.; Chen, H.; Xiong, R.; Zhang, S.; Cheng, H.; Shen, Y.; An, H.; Li, T. Britanin Ameliorates Cerebral Ischemia–Reperfusion Injury by Inducing the Nrf2 Protective Pathway. Antioxid. Redox Signal. 2017, 27, 754–768. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhou, Q.-H.; Xu, M.-B.; Zhou, X.-L.; Zheng, G.-Q. Astragaloside IV for Experimental Focal Cerebral Ischemia: Preclinical Evidence and Possible Mechanisms. Oxid. Med. Cell. Longev. 2017, 2017, 8424326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Y.; Wang, Z.-J.; Sun, D.-M.; Wang, Y.; Xu, P.; Wu, W.-J.; Liu, X.-H.; Zhu, Y.-Z. Novel Therapeutic Effects of Leonurine on Ischemic Stroke: New Mechanisms of BBB Integrity. Oxid. Med. Cell. Longev. 2017, 2017, 7150376. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhang, S.; Zhu, H.; Tian, J. Cornin Ameliorates Cerebral Infarction in Rats by Antioxidant Action and Stabilization of Mitochondrial Function. Phytother. Res. 2010, 24, 547–552. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Zhang, X.; Liu, Z.; Zhang, X.; Dong, L.; Xing, Y.; Wang, C.; Qiao, H.; Zhu, C. Protective Effect of Celastrol in Rat Cerebral Ischemia Model: Down-Regulating p-JNK, Pc-Jun and NF-ΚB. Brain Res. 2012, 1464, 8–13. [Google Scholar] [CrossRef]
- Pasala, P.K.; Shaik, R.A.; Rudrapal, M.; Khan, J.; Alaidarous, M.A.; Khairnar, S.J.; Bendale, A.R.; Naphade, V.D.; Sahoo, R.K.; Zothantluanga, J.H. Cerebroprotective Effect of Aloe Emodin: In Silico and in Vivo Studies. Saudi J. Biol. Sci. 2022, 29, 998–1005. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Du, J.-R.; Zhang, G.-Y.; Kuang, X.; Liu, Y.-X.; Qian, Z.-M.; Wang, C.-Y. Neuroprotective Effect of Z-Ligustilide against Permanent Focal Ischemic Damage in Rats. Biol. Pharm. Bull. 2007, 30, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Kundap, U.P.; Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Plant Derived Phytocompound, Embelin in CNS Disorders: A Systematic Review. Front. Pharmacol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Phua, D.H.; Zosel, A.; Heard, K. Dietary Supplements and Herbal Medicine Toxicities—When to Anticipate Them and How to Manage Them. Int. J. Emerg. Med. 2009, 2, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Fatima, N.; Nayeem, N. Toxic Effects as a Result of Herbal Medicine Intake. In Toxicology—New Aspects to This Scientific Conundrum; InTech Open: London, UK, 2016; pp. 193–207. [Google Scholar]
- Zhang, J.; Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of Herbal Medicines: Challenges and Solutions. Complement. Ther. Med. 2012, 20, 100–106. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Verma, R.; Gupta, J. Challenges and Future Prospects of Herbal Medicine. Int. Res. Med. Health Sci. 2018, 1, 12–15. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Makkar, R.; Sehgal, A.; Sharma, N.; Singh, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S.G. Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders. Molecules 2022, 27, 4970. https://doi.org/10.3390/molecules27154970
Behl T, Makkar R, Sehgal A, Sharma N, Singh S, Albratty M, Najmi A, Meraya AM, Bungau SG. Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders. Molecules. 2022; 27(15):4970. https://doi.org/10.3390/molecules27154970
Chicago/Turabian StyleBehl, Tapan, Rashita Makkar, Aayush Sehgal, Neelam Sharma, Sukhbir Singh, Mohammed Albratty, Asim Najmi, Abdulkarim M. Meraya, and Simona Gabriela Bungau. 2022. "Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders" Molecules 27, no. 15: 4970. https://doi.org/10.3390/molecules27154970
APA StyleBehl, T., Makkar, R., Sehgal, A., Sharma, N., Singh, S., Albratty, M., Najmi, A., Meraya, A. M., & Bungau, S. G. (2022). Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders. Molecules, 27(15), 4970. https://doi.org/10.3390/molecules27154970