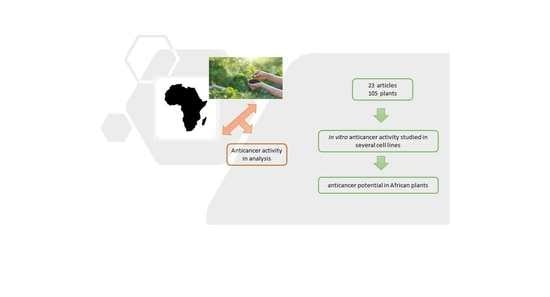
In Vitro Cytotoxic Activity of African Plants: A Review
Abstract
:1. Introduction
2. Results
| Country | Medicinal Plants | Extraction Solvents | Cancer Cell Lines | Results | Reference |
|---|---|---|---|---|---|
| South Africa | Momordica balsamina (leaves) | Acetone | Colorectal carcinoma (HT-29) |
| [14] |
| South Africa | Sutherlandia frutescens (leaves) | 75% (V/V) Ethanol | Colon adenocarcinoma (DLD-1) |
| [15] |
| South Africa | Tulbaghia violacea (leaves) | Methanol Hexane Butanol | Cervical adenocarcinoma (HeLa and ME-180) Breast adenocarcinoma (MDA-MBA-231 and MCF-7) |
| [16] |
| South Africa | Opuntia stricta (cladodes) | Water Acetone Ethanol | Human myeloid leukemia (U937) |
| [17] |
| South Africa | Cotyledon orbiculata (leaves) | Water | Colorectal carcinoma (HCT116) Esophageal adenocarcinoma (OE33) Esophageal squamous carcinoma (KYSE70) |
| [13] |
| South Africa | Asparagus laricinus (cladodes) Senecio asperulus (roots) | Water Methanol Methanol:dichloromethane, 1:1 (V/V) Dichloromethane Hexane | Breast adenocarcinoma (MCF-7) Prostate adenocarcinoma (PC3) |
| [12] |
| South Africa | Kedrostis africana (tuber) | Water Ethanol | Cervical adenocarcinoma (HeLa) |
| [18] |
| South Africa | Centella asiatica (leaves) Curtisia dentata (leaves) Warburgia salutaris (leaves) | Methanol Ethyl acetate Acetone Water | Breast adenocarcinoma (MCF-7) Cervical adenocarcinoma (HeLa) Colorectal adenocarcinoma (Caco-2) Lung adenocarcinoma (A549) |
| [19] |
| Cameroon | Tetrapleura tetraptera (fruits) | Dichloromethane-methanol (1:1) | Resistant CEM/ADR5000 and sensitive CCRF-CEM leukemia cells; Colon cancer HCT116 (p53+/+) cells and t clone HCT116 (p53−/−); Glioblastoma U87MG cells and resistant U87MG.ΔEGFR cells; MDA-MB-231-pcDNA3 breast cancer cells and resistant subline MDA-MB-231-BCRP clone 23 cells; Hepatic carcinoma (HepG2) |
| [20] |
| Cameroon | Fagara tessmannii (bark) | Methanol | Resistant CEM/ADR5000 and sensitive CCRF-CEM leukemia cells; MDA-MB-231-pcDNA3 breast cancer cells and its resistant subline MDA-MB-231-BCRP clone 23 cells; Colon cancer HCT116 (p53+/+) cells and their t clone HCT116 (p53−/−) Glioblastoma U87MG cells and resistant subline U87MG.ΔEGFR cells; Hepatic carcinoma (HepG2) |
| [21] |
| Cameroon | Ficus elastica (wood of aerial roots) Selaginella vogelli (leaves) | Methanol | Cervical adenocarcinoma (HeLa) |
| [22] |
| Cameroon | Sarcocephalus pobeguinii (Roots, fruits, bark, and leaves) | Methanol (leaves/roots/bark) Dichloromethane/methanol (1:2) (fruits) | Breast adenocarcinoma (MCF-7) Cervical adenocarcinoma (HeLa) Colorectal adenocarcinoma (Caco-2) Lung adenocarcinoma (A549) |
| [23] |
| Cameroon | Moringa oleifera (leaves and seeds) | Water | Human lymphoid (Jurkat E6-1) Human leukemia monocytic (THP1) |
| [24] |
| Cameroon | Ananas comosus (peels) Arachis hypogaea (leaves and twigs) Artocarpus heterophyllus (leaves) Camelia sinensis (leaves) Citrus sinensis (fruits) Cola pachycarpa (leaves) Coula edulis (fruits) Curcubita pepo (pericarp) Curcuma longa (rhizomes) Lycopersicon esculentum (twigs and leaves) Mangifera indica (leaves and bark) Myristica fragrans (seeds) Persea Americana (bark) Physalis peruviana (twigs) Psidium guajava (bark) Raphia hookeri (fruits) Rubus fellatae (leaves) Tristemma hirtum (leaves) | Methanol | Resistant CEM/ADR5000 and sensitive CCRF-CEM leukemia cells; MDA-MB-231-pcDNA3 breast cancer cells and their transfectant subline MDA-MB-231-BCRP clone 23; Colon cancer HCT116 (p53+/+) cells and their knockout clone HCT116 (p53−/−); Glioblastoma U87MG cells and their resistant subline U87MG.ΔEGFR |
| [25] |
| Morocco | Calendula arvensis (flowers) | Hexane Methanol Water | Human cancer of myeloid cells |
| [26] |
| Morocco | Ormenis eriolepis (aerial parts) | n-Hexane Methanol | T lymphocyte cell line (Jurkat) Mantle cell lymphoma (Jeko-1) Glioblastoma (LN229) Prostate adenocarcinoma (PC-3) |
| [27] |
| Ghana | Aframomum melegueta (seeds, roots/rhizome) Alstonia boonei (leaves, roots) Baphia nitida (leaves) Desmodium adscendens (leaves, stems) Ficus asperifolia (leaves, stem bark) Mansonia altissima (stem bark) Paullinia pinnata (stem) Spathodea campanulate (leaves, stem bark) Terminalia superba (leaves, stem bark, roots) Triplochiton scleroxylon (leaves, stem bark) | Ethanol–water (1:1) | Hepatic carcinoma (HepG2) Breast adenocarcinoma (MDA-MB-231 and MCF-7) Epidermoid carcinoma (A431) Prostate adenocarcinoma (LNCaP) Lung adenocarcinoma (A549) Gastric adenocarcinoma (AGS) Leukemia (HL-60 and REH) Ewing’s sarcoma (CADO-ES1 and RDES) |
| [28] |
| Ethiopia | Acmella caulirhiza (leaves) Acokanthera schimperi (leaves) Ajuga leucantha (leaves) Aloe debrana (roots) Cineraria abyssinica (leaves) Clausena anisate (leaves) Clematis simensis (leaves) Cleome brachycarpa (leaves) Croton macrostachyus (bark) Dorstenia barnimiana (roots) Euphorbia schimperiana (roots) Gnidia involucrate (roots) Hydrocotyle mannii (leaves) Kalanchoe petitiana (leaves) Kniphofia foliosa (roots) Leonotis ocymifolia (leaves) Pentarrhinum insipidum (roots) Rumex nervosus (roots) Salvia nilotica (whole plant) Sida schimperiana (roots and leaves) Thymus schimperi (leaves) Vernonia auriculifera (leaves) | 80% Methanol | Breast adenocarcinoma (MCF-7) Lung carcinoma (A427) Urinary bladder carcinoma (RT-4) Cervical adenocarcinoma (SiSo) Large cell lung carcinoma (LCLC-103H) Pancreatic carcinoma (DAN-G) Ovarian carcinoma (A2780) Esophageal squamous carcinoma (KYSE-70) Acute myeloid leukemia (HL-60) Human myeloid leukemia (U-937) |
| [29] |
| Côte d’Ivoire | Bridelia ferruginea (leaves and stem barks) | Methanol Ethyl acetate Water | Colorectal carcinoma (HCT116) |
| [30] |
| Egypt | Brassica nigra (seeds) | 50% (V/V) Ethanol | Human non-small cell lung carcinoma (A549 and H1299) |
| [31] |
| Burkina Faso | Lantana ukambensis (whole plant) | Dichloromethane | Colorectal carcinoma (HCT-116 and HT-29) |
| [32] |
| Algeria | Heliotropium bacciferum (aerial parts) | Chloroform Methanol | Colorectal carcinoma (HCT116) Colorectal adenocarcinoma (DLD1) |
| [33] |
| Kenya | Abrus precatorius Aeschynomene abyssinica Albizia gumífera, Aloe volkensii Bridelia micrantha Conyza sumatrensis Croton macrostachyus Cyphostemma serpens Entada abyssinica, Ficus thonningii Fuerstia africana, Futumia africana Harungana madagascariensis Ipomoea cairica Microglossa pyrifolia Momordica foetida Moringa oleífera Ocimum gratissimum, Olea hotch Phyllanthus sapialis, P. fischeri Prunus africana Psydrax schimperiana Rotheca myricoides Senna didymobotyra Shirakiopsis elliptica, Sida rhombifolia Spathodea campanulate Synsepalum cerasiferum Tragia brevipes, Trichilia emetica Triumfetta rhomboidei, Vernonia lasiopus Zanthoxylum rubescens, Z. gilletii | Dichloromethane/methanol (organic) and water | Sensitive and drug-resistant human cancer cell lines: Sensitive CCRF-CEM and multidrug-resistant P glycoprotein-overexpressing CEM/ADR5000; Wild-type HCT116 (p53+/+) and knockout HCT116 (p53−/−) colon cancer cells; Breast cancer cells transduced with control vector (MDAMB-231-pcDNA3) or with cDNA for the breast cancer resistance protein BCRP (MDA-MB-231-BCRP clone 23) Wild-type U87MG cells and U87MG glioblastoma multiforme cells transfected with an expression vector harboring an epidermal growth factor receptor (EGFR) gene with a genomic deletion of exons 2 through 7 (U87MG.ΔEGFR) |
| [34] |
3. Discussion
3.1. Cytotoxicity
3.2. Mechanisms of Action
3.3. Effect of the Extract Solvents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. CMLS 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Kuete, V.; Efferth, T. Potential of Central, Eastern and Western Africa Medicinal Plants for Cancer Therapy: Spotlight on Resistant Cells and Molecular Targets. Front. Pharmacol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Singh, S.; Skvortsova, I.; Kumar, V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr. Med. Chem. 2017, 24, 4729–4752. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [Green Version]
- Mbele, M.; Hull, R.; Dlamini, Z. African medicinal plants and their derivatives: Current efforts towards potential anti-cancer drugs. Exp. Mol. Pathol. 2017, 103, 121–134. [Google Scholar] [CrossRef]
- Sharma, R.; Aashima; Nanda, M.; Fronterre, C.; Sewagudde, P.; Ssentongo, A.E.; Yenney, K.; Arhin, N.D.; Oh, J.; Amponsah-Manu, F.; et al. Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates From GLOBOCAN 2020. Front. Public Health 2022, 10, 839835. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [Green Version]
- Mfengwana, P.H.; Mashele, S.S.; Manduna, I.T. Cytotoxicity and cell cycle analysis of Asparagus laricinus Burch. and Senecio asperulus DC. on breast and prostate cancer cell lines. Heliyon 2019, 5, e01666. [Google Scholar] [CrossRef] [Green Version]
- Makhafola, T.J.; Mbele, M.; Yacqub-Usman, K.; Hendren, A.; Haigh, D.B.; Blackley, Z.; Meyer, M.; Mongan, N.P.; Bates, D.O.; Dlamini, Z. Apoptosis in Cancer Cells Is Induced by Alternative Splicing of hnRNPA2/B1 Through Splicing of Bcl-x, a Mechanism that Can Be Stimulated by an Extract of the South African Medicinal Plant, Cotyledon orbiculata. Front. Oncol. 2020, 10, 547392. [Google Scholar] [CrossRef]
- Serala, K.; Steenkamp, P.; Mampuru, L.; Prince, S.; Poopedi, K.; Mbazima, V. In vitro antimetastatic activity of Momordica balsamina crude acetone extract in HT-29 human colon cancer cells. Environ. Toxicol. 2021, 36, 2196–2205. [Google Scholar] [CrossRef]
- Zonyane, S.; Fawole, O.A.; la Grange, C.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations. Antioxidants 2020, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Motadi, L.R.; Choene, M.S.; Mthembu, N.N. Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci. Rep. 2020, 10, 12924. [Google Scholar] [CrossRef]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical composition, antioxidant, antiinflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS ONE 2019, 14, e0209682. [Google Scholar] [CrossRef] [Green Version]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.). Cogn. Heliyon 2018, 4, e00810. [Google Scholar] [CrossRef] [Green Version]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) CA Sm-medicinal plants used in South Africa. BMC Complement. Altern. Med. 2018, 18, 315. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Chi, G.F.; Bonsou, I.N.; Ombito, J.O.; Yeboah, S.O.; Kuete, V.; Efferth, T. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 2021, 267, 113632. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Damen, F.; Çelik, İ.; Tane, P.; Kuete, V.; Efferth, T. Cytotoxicity of the crude extract and constituents of the bark of Fagara tessmannii towards multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 2019, 235, 28–37. [Google Scholar] [CrossRef]
- Mbosso Teinkela, J.E.; Siwe Noundou, X.; Nguemfo, E.L.; Meyer, F.; Wintjens, R.; Isaacs, M.; Mpondo Mpondo, A.E.; Hoppe, H.C.; Krause, R.; Azebaze, A. Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: An antimalarial, antitrypanosomal and cytotoxity evaluation. Saudi J. Biol. Sci. 2018, 25, 117–122. [Google Scholar] [CrossRef]
- Mfotie Njoya, E.; Munvera, A.M.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J. Phytochemical analysis with free radical scavenging, nitric oxide inhibition and antiproliferative activity of Sarcocephalus pobeguinii extracts. BMC Complement. Altern. Med. 2017, 17, 199. [Google Scholar] [CrossRef]
- Potestà, M.; Minutolo, A.; Gismondi, A.; Canuti, L.; Kenzo, M.; Roglia, V.; Macchi, F.; Grelli, S.; Canini, A.; Colizzi, V.; et al. Cytotoxic and apoptotic effects of different extracts of Moringa oleifera Lam on lymphoid and monocytoid cells. Exp. Ther. Med. 2019, 18, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Mbaveng, A.T.; Manekeng, H.T.; Nguenang, G.S.; Dzotam, J.K.; Kuete, V.; Efferth, T. Cytotoxicity of 18 Cameroonian medicinal plants against drug sensitive and multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 2018, 222, 21–33. [Google Scholar] [CrossRef]
- Abudunia, A.M.; Marmouzi, I.; Faouzi, M.E.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Anticandidal, antibacterial, cytotoxic and antioxidant activities of Calendula arvensis flowers. J. Mycol. Med. 2017, 2, 90–97. [Google Scholar] [CrossRef]
- Belayachi, L.; Aceves-Luquero, C.; Merghoub, N.; Fernández de Mattos, S.; Amzazi, S.; Villalonga, P.; Bakri, Y. Induction of cell cycle arrest and apoptosis by Ormenis eriolepis a Morrocan endemic plant in various human cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 356–373. [Google Scholar] [CrossRef] [Green Version]
- Spiegler, V.; Greiffer, L.; Jacobtorweihen, J.; Asase, A.; Lanvers-Kaminsky, C.; Hempel, G.; Agyare, C.; Hensel, A. In vitro screening of plant extracts traditionally used as cancer remedies in Ghana–15-Hydroxyangustilobine A as the active principle in Alstonia boonei leaves. J. Ethnopharmacol. 2021, 265, 113359. [Google Scholar] [CrossRef]
- Tesfaye, S.; Braun, H.; Asres, K.; Engidawork, E.; Belete, A.; Muhammad, I.; Schulze, C.; Schultze, N.; Guenther, S.; Bednarski, P.J. Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer; Part 3: Selective Cytotoxic Activity of 22 Plants against Human Cancer Cell Lines. Molecules 2021, 26, 3658. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Jugreet, S.; Sinan, K.I.; Zengin, G.; Ak, G.; Ceylan, R.; Jekő, J.; Cziáky, Z.; Angelini, P.; Angeles Flores, G.; et al. Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.-A Native Tropical African Medicinal Plant. Antibiotics 2021, 10, 223. [Google Scholar] [CrossRef]
- Ahmed, A.G.; Hussein, U.K.; Ahmed, A.E.; Kim, K.M.; Mahmoud, H.M.; Hammouda, O.; Jang, K.Y.; Bishayee, A. Mustard Seed (Brassica nigra) Extract Exhibits Antiproliferative Effect against Human Lung Cancer Cells through Differential Regulation of Apoptosis, Cell Cycle, Migration, and Invasion. Molecules 2020, 25, 2069. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.R.; Luo, Y.; Elkington, B.; He, T.C.; Wang, C.Z.; Yuan, C.S. Cytotoxicity and Preliminary Analysis of the Pro-apoptotic and Cell Cycle Arrest Effects of Lantana ukambensis Against Colorectal Cancer Cells. Int. J. Appl. Biol. Pharm. 2020, 11, 170–187. [Google Scholar] [PubMed]
- Aïssaoui, H.; Mencherini, T.; Esposito, T.; De Tommasi, N.; Gazzerro, P.; Benayache, S.; Benayache, F.; Mekkiou, R. Heliotropium bacciferum Forssk. (Boraginaceae) extracts: Chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat. Prod. Res. 2019, 33, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Ochwang’I, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Kiama, S.G.; Efferth, T. Cytotoxic activity of medicinal plants of the Kakamega County (Kenya) against drug-sensitive and multidrug-resistant cancer cells. J. Ethnopharmacol. 2018, 215, 233–240. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Abdellateif, M.S.; Zekri, A.N. Cancer in Africa: Is It a Genetic or Environmental Health Problem? Front. Oncol. 2020, 10, 604214. [Google Scholar] [CrossRef]
- GLOBOCON. Southern Africa-Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 25 July 2021).
- World Health Organization. Regional Office for Africa. Cancer. Available online: https://www.afro.who.int/health-topics/cancer (accessed on 25 June 2021).
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001. [Google Scholar]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Ayoub, I.M.; El-Shazly, M.; Lu, M.C.; Singaba, A.N.B. Antimicrobial and cytotoxic activities of the crude extracts of Dietes bicolor leaves, flowers and rhizomes. S. Afr. J. Bot. 2014, 95, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Badmus, J.A.; Ekpo, O.E.; Hussein, A.A.; Meyer, M.; Hiss, D.C. Antiproliferative and apoptosis induction potential of the methanolic leaf extract of Holarrhena floribunda (G. Don). J. Evid.-Based Complement. Altern. Med. 2015, 2015, 756482. [Google Scholar] [CrossRef]
- Narrima, P.; Looi, C.Y.; Mohd, M.A.; Ali, H.M. Apoptosis activity of Persea declinata (Bl.) kosterm bark methanolic crude extract. World Academy of Science, Engineering and Technology. Int. J. Med. Health Biomed. Bioeng. Pharm. Eng. 2014, 8, 664–669. [Google Scholar]
- Hu, Y.; Sun, Z.; Deng, J.; Hu, B.; Yan, W.; Wei, H.; Jiang, J. Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumor Biol. 2017, 39, 1010428317694318. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bao, C.; Ma, Z.; Xu, B.; Ying, X.; Liu, X.; Zhang, X. Perfluorooctanoic acid stimulates ovarian cancer cell migration, invasion via ERK/NF-κB/MMP-2/-9 pathway. Toxicol. Lett. 2018, 294, 44–50. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Xu, P.; Miao, C.; Zeng, X.; Cui, X.; Lu, C.; Xie, H.; Yin, H.; Chen, F.; et al. Perfluorooctanoic acid stimulates breast cancer cells invasion and up-regulates matrix metalloproteinase-2/-9 expression mediated by activating NF-κB. Toxicol. Lett. 2014, 229, 118–125. [Google Scholar] [CrossRef]
- Chou, Y.C.; Sheu, J.R.; Chung, C.L.; Chen, C.Y.; Lin, F.L.; Hsu, M.J.; Kuo, Y.H.; Hsiao, G. Nuclear-targeted inhibition of NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl caffeamide in human monocytic cells. Chem. Biol. Interact. 2010, 184, 403–412. [Google Scholar] [CrossRef]
- Naidoo, D.B.; Chuturgoon, A.A.; Phulukdaree, A.; Guruprasad, K.P.; Satyamoorthy, K.; Sewram, V. Centella asiatica modulates cancer cachexia associated inflammatory cytokines and cell death in leukaemic THP-1 cells and peripheral blood mononuclear cells (PBMC’s). BMC Complement. Altern. Med. 2017, 17, 377. [Google Scholar] [CrossRef]
- Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.Á.; Pinho, C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules 2022, 27, 4989. https://doi.org/10.3390/molecules27154989
Canga I, Vita P, Oliveira AI, Castro MÁ, Pinho C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules. 2022; 27(15):4989. https://doi.org/10.3390/molecules27154989
Chicago/Turabian StyleCanga, Isabel, Pedro Vita, Ana Isabel Oliveira, María Ángeles Castro, and Cláudia Pinho. 2022. "In Vitro Cytotoxic Activity of African Plants: A Review" Molecules 27, no. 15: 4989. https://doi.org/10.3390/molecules27154989
APA StyleCanga, I., Vita, P., Oliveira, A. I., Castro, M. Á., & Pinho, C. (2022). In Vitro Cytotoxic Activity of African Plants: A Review. Molecules, 27(15), 4989. https://doi.org/10.3390/molecules27154989