Effect of Bauhinia monandra Kurz Leaf Preparations on Embryonic Stages and Adult Snails of Biomphalaria glabrata (Say, 1818), Schistosoma mansoni Cercariae and Toxicity in Artemia salina
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of the Composition of Saline Extract and Fraction of B. monandra Were Assessed
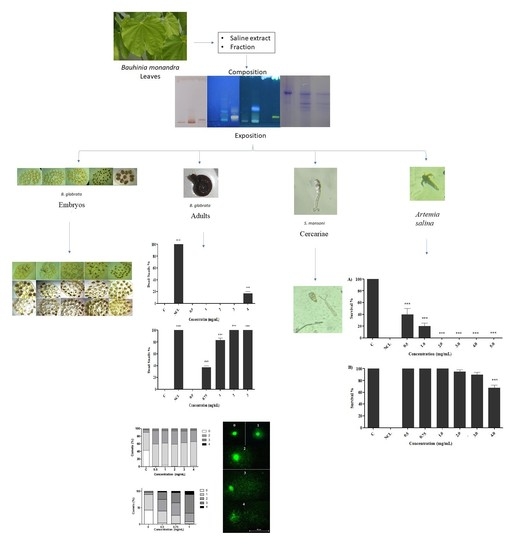
2.2. Effect of Preparations on B. glabrata Embryos
2.3. Effect of Preparations on Adult Snails of B. glabrata
2.4. Effect of Preparations on B. glabrata Hemocytes
2.5. Comet Test
2.6. Evaluation of Toxicity in S. mansoni Cercariae
2.7. Artemia Salina Acute Test
3. Materials and Methods
3.1. Plant
3.2. Animals
3.3. Preparation of Saline Extract and Fraction of B. monandra
3.4. Protein Concentration and Hemagglutinating Activity
3.5. Phytochemical Screening
3.6. High Performance Liquid Chromatography (HPLC) Analysis
3.7. Toxicity of Preparations in Embryonic Stages of B. glabrata
3.8. Toxicity, Fertility and Fecundity Analysis in Adult Snails of B. glabrata
3.9. Evaluation of Cytotoxicity in B. glabrata Snail Hemocytes
3.10. Evaluation of Genotoxicity by Comet Assay
3.11. Fluorescence Microscopy Analysis
3.12. Activity in S. mansoni Cercariae
3.13. Environmental Ecotoxicity Assessment Using A. salina
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Faria, R.X.; Rocha, L.M.; Souza, E.P.B.S.S.; Almeida, F.B.; Fernandes, C.P.; Santos, J.A.A. Molluscicidal activity of Manilkara subsericea (Mart.) dubard on Biomphalaria glabrata (Say, 1818). Acta Trop. 2018, 178, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.; Fernandes, Y.M.L.; Silva, C.R.; Costa-Junior, L.M.; Figueiredo, P.L.B.; Monteiro, O.S.; Maia, J.G.S.; da Rocha, C.Q. Anthelmintic evaluation and essential oils composition of Hyptis dilatata Benth. and Mesosphaerum suaveolens Kuntze from the Brazilian Amazon. Acta Trop. 2022, 228, 106321. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Promotion and Development of Traditional Medicine: Report of a WHO Meeting, Geneva, Switzerland, 28 November to 2 December 1977; Technical Report Series 622; WHO: Geneva, Switzerland, 1978; p. 41. [Google Scholar]
- Ribeiro, E.C.G.; Leite, J.A.C.; Luz, T.R.S.A.; Silveira, D.P.B.; Bezerra, S.A.; Frazão, G.C.C.G.; Pereira, L.P.L.A.; Guimarães dos Santos, E.G.; Ribeiro Filho, P.R.C.F.; Soares, A.M.S.; et al. Molluscicidal activity of monoterpenes and their effects on inhibition of acetylcholinesterase activity on Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Acta Trop. 2021, 223, 106089. [Google Scholar] [CrossRef]
- Schalla, V.T.; Vasconcellos, M.C.; Rocha, R.S.; Souza, C.P.; Mendes, N.M. The control of the schistosome-transmitting snail Biomphalaria glabrata by the plant Molluscicide Euphorbia splendens var. hislopii (syn milli Des. Moul): A longitudinal field study in an endemic area in Brazil. Acta Trop. 2001, 79, 165–170. [Google Scholar] [CrossRef]
- Pereira, L.P.L.A.; Ribeiro, E.C.G.; Brito, M.C.A.; Araruna, F.O.S.; Araruna, F.B.; Leite, J.A.C.; Silveira, D.P.B.; Oliveira, T.M.; Cantanhede, S.P.D.; Firmo, W.D.C.A.; et al. Molluscicidal and cercaricidal activities of the essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants: Implications for the control of schistosomiasis. Acta Trop. 2022, 230, 106393. [Google Scholar] [CrossRef]
- World Health Organization. Schistosomiasis. W.H.O. 2022. Available online: http://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 8 May 2022).
- Scholte, R.G.; Gosoniu, L.; Malone, J.B.; Chammartin, F.; Utzinger, J.; Vounatsou, P. Predictive risk mapping of schistosomiasis in Brazil using Bayesian geostatistical models. Acta Trop. 2014, 132, 57–63. [Google Scholar] [CrossRef]
- Paz, W.S.D.; Gomes, D.S.; Ramos, R.E.S.; Cirilo, T.M.; Santos, I.G.A.; Ribeiro, C.J.N.; Araújo, K.C.G.M.; Jesus, A.M.R.; Santos, A.D.D.; Bezerra-Santos, M. Spatiotemporal clusters of schistosomiasis mortality and association with social determinants of health in the Northeast region of Brazil (1980–2017). Acta Trop. 2020, 212, 105668. [Google Scholar] [CrossRef]
- Coelho, P.M.Z.; Caldeira, R.L. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect. Dis. Poverty. 2016, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Paz, W.S.; Duthie, M.S.; Jesus, A.R.; Machado de Araújo, K.C.G.; Santos, A.D.; Bezerra-Santos, M. Population-based, spatiotemporal modeling of social risk factors and mortality from schistosomiasis in Brazil between 1999 and 2018. Acta Trop. 2021, 218, 105897. [Google Scholar] [CrossRef]
- Silva, H.A.M.F.; Siqueira, W.N.; Sá, J.L.F.; Silva, L.R.S.; Martins, M.C.B.; Aires, A.L.; Amâncio, F.F.; Pereira, E.C.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; et al. Laboratory assessment of divaricatic acid against Biomphalaria glabrata and Schistosoma mansoni cercariae. Acta Trop. 2018, 178, 97–102. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Field use of molluscicides in schistosomiasis control programmes: An operational manual for programme managers. W.H.O. 2017, 40. Available online: https://apps.who.int/iris/handle/10665/254641 (accessed on 18 April 2022).
- Albuquerque, L.P.; Pontual, E.V.; Santana, G.M.S.; Silva, L.R.S.; Aguiar, J.S.; Coelho, L.C.B.B.; Rêgo, M.J.B.M.; Pitta, M.G.R.; Silva, T.G.; Melo, A.M.M.A.; et al. Toxic effects of Microgramma vacciniifolia rhizome lectin on Artemia salina, human cells, and the schistosomiasis vector Biomphalaria glabrata. Acta Trop. 2014, 138, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.S.F.; Araujo, T.F.S.; Silva, C.B.; Campos, J.K.L.; Paiva, P.M.G.; Napoleão, T.H.; Albuquerque, P.B.S.; Lima, V.L.M.; Coelho, L.C.B.B. Evaluation of toxicity on mice and artemicidal activity of Bauhinia monandra leaf lectin (BmoLL). Theory Appl. Microbiol. Biotechnol. 2019, 1, 44–52. [Google Scholar] [CrossRef]
- Silva, H.A.M.F.; Sá, J.L.F.; Siqueira, W.N.; Lima, M.V.; Martins, M.C.B.; Aires, A.L.; Albuquerque, M.C.P.A.; Falcão, E.P.D.S.; Buril, M.L.L.; Pereira, E.C.; et al. Toxicological effects of Ramalina aspera (lichen) on Biomphalaria glabrata snails and Schistosoma mansoni cercariae. Acta Trop. 2019, 196, 172–179. [Google Scholar] [CrossRef]
- Nworie, K.M.; Okorie, N.A. Phytochemicals distribution and antioxidant potential of Bauhinia monandra (Linn.) leaves extract. Res. J. Med. Plants. 2018, 12, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Kurz, W.S. Natural History. J. Asiat. Soc. Bengal. 1873, 42, 1–73. [Google Scholar]
- Cagliari, R.; Kremer, F.S.; Pinto, L.D.S. Bauhinia lectins: Biochemical properties and biotechnological applications. Int. J. Biol. Macromol. 2018, 19, 811–820. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Santos, N.R.M.; Cabrera, M.P.; Ferreira, S.A.O.; Raposo, B.L.; Napoleao, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Cabral Filho, P.E.; Fontes, A.; et al. Bauhinia monandra leaf lectin (BmoLL) conjugated with quantum dots as fluorescent nanoprobes for biological studies: Application to red blood cell. Methods Appl. Fluoresc. 2020, 8, 7. [Google Scholar] [CrossRef]
- Coelho, L.C.B.B.; Silva, M.B.R. Simple method to purify milligram quantities of the galactose-specific lectin from the leaves of Bauhinia monandra. Phytochem. Anal. 2000, 11, 295–300. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Freire, M.G.M.; Silva, M.B.R.; Coelho, L.C.B.B. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007, 146, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.A.F.; Silva, L.C.N.; Correia, M.T.S.; Araújo, J.M.; Coelho, L.C.B.B. Endophytic microorganisms from Bauhinia monandra leaves: Isolation, antimicrobial activities and interaction with galactose-specific lectin BmoLL. Afric. J. Microbiol. Res. 2016, 10, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Araujo-Melo, R.O.; Souza, I.F.A.C.; Oliveira, C.V.J.; Araujo, J.M.; Sena, K.X.F.R.; Coelho, L.C.B.B. Isolation and identification of endophyte microorganisms from Bauhinia monandra leaves, mainly actinobacteria. Biotechnol. J. Int. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Singh, K.L.; Singh, D.K.; Singh, V.K. Characterization of the molluscicidal activity of Bauhinia variegata and Mimusops elengi plant extracts against the fascista vector Lymnaea acuminata. Rev. Inst. Med. Trop. S. Paulo 2012, 54, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar] [CrossRef]
- Piana, M.; Zadra, M.; Brum, T.F.; Boligon, A.A.; Gonçalves, A.F.K.; Cruz, R.C.; Freitas, R.B.; Canto, G.S.; Athayde, M.L. Analysis of Rutin in the Extract and Gel of Viola tricolor. J. Chromatogr. Sci. 2013, 51, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.A.; Passos, A.M.P.R.; Andrade, F.C.; Camargo, E.A.; Estevam, C.S.; Santos, M.R.V.; Thomazzi, S.M. Antinociceptive and anti-inflammatory effects of Caesalpinia pyramidalis in rodents. Rev. Bras. Farmacogn. 2011, 21, 6. [Google Scholar] [CrossRef] [Green Version]
- Pena, R.V.; Machado, R.C.; Caixeta, M.B.; Araújo, P.S.; Oliveira, E.C.; Silva, S.M.; Rocha, T.L. Lauric acid bilayer-functionalized iron oxide nanoparticles disrupt early development of freshwater snail Biomphalaria glabrata (Say, 1818). Acta Trop. 2022, 229, 106362. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.C.; Geraldino, B.R.; Coelho, D.R.; Carvalho, R.R.; Paumgartten, F.J.R. Comparative toxicity of Euphorbia milii latex and synthetic molluscicides to Biomphalaria glabrata embryos. Chemosphere. 2010, 8, 219–227. [Google Scholar] [CrossRef]
- Rocha-Filho, C.A.A.; Albuquerque, L.P.; Silva, L.R.S.; Silva, P.C.B.; Coelho, L.C.C.B.; Navarro, D.M.A.F.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; Napoleão, T.H.; Pontual, E.V.; et al. Assessment of toxicity of Moringa oleifera flower extract to Biomphalaria glabrata, Schistosoma mansoni and Artemia salina. Chemosphere. 2015, 132, 188–192. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Silva, L.R.S.; Siqueira, W.N.; Fonseca, C.S.M.; Silva, N.H.; Melo, A.M.M.A.; Martins, M.C.B.; Lima, V.L.M. Toxicity of usnic acid from Cladonia substellata (Lichen) to embryos and adults of Biomphalaria glabrata. Acta Trop. 2018, 179, 39–43. [Google Scholar] [CrossRef]
- Araújo, P.S.; Caixeta, M.B.; Brito, R.D.S.; Gonçalves, B.B.; Silva, S.M.; Lima, E.C.O.; Silva, L.D.; Bezerra, J.C.B.; Rocha, T.L. Molluscicidal activity of polyvinylpyrrolidone (PVP)-functionalized silver nanoparticles to Biomphalaria glabrata: Implications for control of intermediate host snail of Schistosoma mansoni. Acta Trop. 2020, 211, 105644. [Google Scholar] [CrossRef] [PubMed]
- Burić, P.; Jakšić, Ž.; Štajner, L.; Sikirić, M.D.; Jurašin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean Sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, J.J.; Araújo, H.D.A.; Aguiar, T.W.A.; Ferreira, S.A.O.; Lima, M.V.; Pereira, D.R.; Ferreira, M.R.A.; Soares, L.A.L.; Melo, A.M.M.A.; Albuquerque, M.C.P.A.; et al. Toxic, cytotoxic and genotoxic effect of saline extract and fraction of Parkia pendula seeds in the developmental stages of Biomphalaria glabrata (Say 1818—Intermediate host) and cercaricide activity against the infectious agent of schistosomiasis. Acta Trop. 2022, 228, 106312. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, H.A.M.F.; Siqueira, W.N.; Santos, V.H.B.; Lima, M.V.; Silva Júnior, J.G.; Silva, N.H.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; Aires, A.L.; et al. Sublethal concentrations of usnic acid potassium salt impairs physiological parameters of Biomphalaria glabrata (Say, 1818) (Pulmonata: Planorbidae) infected and not infected with Schistosoma mansoni. Acta Trop. 2021, 222, 106067. [Google Scholar] [CrossRef]
- Oyeyemi, O.T. Application of nanotized formulation in the control of snail intermediate hosts of schistosomes. Acta Trop. 2021, 220, 105945. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.P.L.A.; Ribeiro, E.C.G.; Brito, M.C.A.; Silveira, D.P.B.; Araruna, F.O.S.; Araruna, F.B.; Leite, J.A.C.; Dias, A.A.S.; Firmo, W.D.C.A.; Borges, M.O.D.R.; et al. Essential oils as molluscicidal agents against schistosomiasis transmitting snails—A review. Acta Trop. 2020, 209, 105489. [Google Scholar] [CrossRef]
- Kiros, G.; Erko, B.; Giday, M.; Mekonnen, Y. Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BCM Res. Notes. 2014, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, I.A.T.A.; Sá, J.L.F.; Lima, M.V.; Veras, S.T.S.; Aguiar, J.C.R.O.F.; Aires, A.L.; Albuquerque, M.C.P.A.; Da Silva, M.V.; Melo, A.M.M.A.; Navarro, D.M.A.F.; et al. Toxic effect of Croton rudolphianus leaf essential oil against Biomphalaria glabrata, Schistosoma mansoni cercariae and Artemia salina. Acta Trop. 2021, 223, 106102. [Google Scholar] [CrossRef]
- Chifundera, K.; Baluku, B.; Mashimango, B. Phytochemical screening and molluscicidal potency of same zairean medicinal plants. Pharmacol. Res. 1993, 28, 333–340. [Google Scholar] [CrossRef]
- Hymete, A.; Iversen, T.H.; Rohloff, J.; Erko, B. Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine 2005, 12, 675–679. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Bakry, F.A. Assessment of the molluscicidal impact of extracted chlorophyllin on some biochemical parameters in the nervous tissue and histological changes in Biomphalaria alexandrina and Lymnaea natalensis snails. Invert. Neurosci. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, W.; Dong, X.; Hu, R.; Nan, X. Molluscicidal activity of cardiac glycosides from Nerium indicum against Pomacea canaliculata and its implications for the mechanisms of toxicity. Environ. Toxicol. Pharmacol. 2011, 32, 226–232. [Google Scholar] [CrossRef]
- Augusto, R.C.; Friani, G.; Vasconcellos, M.C.; Rodrigues, M.L.A.; Mello-Silva, C.C. Schistosoma mansoni: Phytochemical effect on aquatic life cycle. J. Vet. Med. 2015, 5, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Jaqueline, L.M.; Silva, K.R.; Paula, L.A.L.; Cunha, W.R.; Ramos, S.B.; Rodrigues, V.; Cabral, F.J.; Magalhaes, L.G. Molluscicidal and cercaricidal activities of curcumin on Biomphalaria glabrata and Schistosoma mansoni cercariae. Pest. Manag. Sci. 2020, 76, 1228–1234. [Google Scholar] [CrossRef]
- Rizk, M.Z.; Metwally, N.S.; Hamed, M.A.; Mohamed, A.M. Correlation between steroid sex hormones, egg laying capacity and cercarial shedding in Biomphalaria alexandrina snails after treatment with Haplophyllum tuberculatum. Exp. Parasitol. 2012, 132, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Fioroni, P.; Stroben, E.; Markert, B. Tributyltin (TBT) effects on Ocinebrina aciculata (Gastropoda: Muricidae): Imposex development, sterilization, sex change and population decline. Sci. Total Environ. 1996, 188, 205–223. [Google Scholar] [CrossRef]
- Lima, M.V.; Pereira, M.I.A.; Cabral Filho, P.E.; Siqueira, W.N.; Silva, H.A.M.F.; França, E.J.; Santos, B.S.; Melo, A.M.M.A.; Fontes, A. Studies on toxicity of suspensions of CdTe quantum dots to Biomphalaria Glabrata Mollusks. Environ. Toxicol. Chem. 2019, 38, 2128–2136. [Google Scholar] [CrossRef]
- Parry, H.E.; Pipe, R.K. Interactive effects of temperature and copper on immunocompetence and disease susceptibility in mussels (Mytilus edulis). Aquat. Toxicol. 2004, 20, 311–325. [Google Scholar] [CrossRef]
- Perez, D.G.; Fontanetti, C.S. Hemocitical responses to environmental stress in invertebrates: A review. Environ. Monit. Assess. 2011, 177, 437–447. [Google Scholar] [CrossRef]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate immune memory in invertebrate metazoans: A critical appraisal. Front. Immunol. 2018, 9, 17. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Impact of phthalates and bisphenols plasticizers on haemocyte immune function of aquatic invertebrates: A review on physiological, biochemical, and genomic aspects. J. Hazard. Mater. 2021, 419, 9. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H. “Where, O death, is thy sting?” A brief review of apoptosis biology. Mol. Neurobiol. 2010, 42, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Afanasieva, K.; Sivolob, A. Physical principles and new applications of comet assay. Biophys. Chem. 2018, 238, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.N.; França, E.J.; Pereira, D.R.; Lima, M.V.; Silva, H.A.M.F.; Araújo, H.D.A.; Sá, J.L.F.; Melo, A.M.M.A. Study of genotoxic and cytotoxic effects after acute and chronic exposures to industrial sewage sludge on Biomphalaria glabrata hemocytes. Chemosphere 2020, 249, 126218. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Singh, M.B.; Misra, M.K.; Bhalla, P.L.; Tuteja, R. Molecular mechanisms of DNA damage and repair: Progress in plants. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 337–397. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.; Cabral-De-Mello, D.C.; Marin-Morales, M.A. Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium. Ecotoxicol. Environ. Saf. 2015, 120, 174–183. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Ahmed, A.K.; Bakry, F.A.; Rabei, I.; Abdel-Ghaffar, F. Toxicological impact of butralin, glyphosate-isopropylammonium and pendimethalin herbicides on physiological parameters of Biomphalaria alexandrina snails. Mol. Res. 2019, 39, 224–233. [Google Scholar] [CrossRef]
- Siqueira, W.N.; França, E.J.; Pereira, D.R.; Lima, M.V.; Silva, H.A.M.F.; Sá, J.L.F.; Araújo, H.D.A.; Melo, A.M.M.A. Toxicity and genotoxicity of domestic sewage sludge in the freshwater snail Biomphalaria glabrata (Say, 1818). Environ. Sci. Pollut. Res. Int. 2021, 28, 69343–69353. [Google Scholar] [CrossRef]
- Morad, M.Y.; El-Sayed, H.; Elhenawy, A.A.; Korany, S.M.; Aloufi, A.S.; Ibrahim, A.M. Myco-synthesized molluscicidal and larvicidal selenium nanoparticles: A new strategy to control Biomphalaria alexandrina snails and larvae of Schistosoma mansoni with an in silico study on induced oxidative stress. J. Fungi. 2022, 8, 262. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Ghoname, S.I. Molluscicida impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Exp. Parasitol. 2018, 192, 36–41. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Tarrab-Hazdai, R.; Meshulam, H.; Arnon, R. Effect of phosphonium salts and phosphoranes on the acetylcholinesterase activity and on the viability of Schistosoma mansoni parasites. Int. J. Immunopharmacol. 1984, 6, 619–627. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Melo, A.M.M.A.; Siqueira, W.N.; Martins, M.C.B.; Aires, A.L.; Albuquerque, M.C.P.A.; Silva, N.H.; Lima, V.L.M. Potassium usnate toxicity against embryonic stages of the snail Biomphalaria glabrata and Schistosoma mansoni cercariae. Acta Trop. 2018, 188, 132–137. [Google Scholar] [CrossRef]
- World Health Organization. Research priorities for Helminth infections. Technical report on the TDR disease reference group on Helminth infections, in: World Health Organization. Tech. Rep. Ref. Ser. 2012, 972, 79. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Paiva, P.M.G.; Coelho, L.C.B.B. Purification and partial characterization of two lectins isoforms from Cratyliamollis Mart. (Camaratu Bean). Biochem. Biotechn. 1992, 36, 113–118. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, M.B.; Araújo, P.S.; Pereira, A.C.; Tallarico, L.F.; Rocha, T.L. Biomphalaria embryotoxicity test (BET): 60 years of research crossing boundaries for developing standard protocols. Sci. Total Environ. 2022, 833, 155211. [Google Scholar] [CrossRef]
- Pavlica, M.; Klobučar, G.; Vetma, N.; Erben, R.; Papeš, D. Detection of micronuclei in haemocytes of zebra mussel and great ramshorn snail exposed to pentachlorophenol. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 465, 145–150. [Google Scholar] [CrossRef]
- Singh, N.P.; Mccoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivão, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, L.R.S.; Siqueira, W.N.; Fonseca, C.S.M.; Nicacio, H.S.; Melo, A.M.M.A.; Martins, M.C.B.M.; Lima, V.L.M.L. 2018. Dataset on usnic acid from Cladonia substellata Vainio (Lichen) schistosomiasis mansoni’s vector control and environmental toxicity. Data Brief 2008, 17, 288–291. [Google Scholar] [CrossRef] [PubMed]








| Experimental Groups | Unviable by Test % | ||||
|---|---|---|---|---|---|
| Embryonic Stages | |||||
| Blastula | Gastrula | Trochophore | Veliger | Hippo Stage | |
| H2O | 1.3 ± 0.5 | 0.3 ± 0.5 | 1 ± 1 | 1 ± 1 | 1.6 ± 0.6 |
| NCL | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| Saline Extract (mg/mL) | Blastula | Gastrula | Trochophore | Veliger | Hippo Stage |
| 0.0125 | 25.3 ± 7.0 b | 14.0 ± 3.4 a | 0.6 ± 0.5 | 3.6 ± 1.5 | 1.6 ± 2.0 |
| 0.025 | 33.7 ± 2.0 c | 37.0 ± 13.6 c | 13.6 ± 6.4 c | 11.0 ± 2.0 | 1.3 ± 0.5 |
| 0.05 | 61.0 ± 12.0 c | 55.0 ± 5.3 c | 29.6 ± 3.5 c | 14.6 ± 4.6 b | 5.3 ± 2.0 |
| 0.1 | 87.7 ± 1.5 c | 71.3 ± 2.5 c | 63.0 ± 4.0 c | 23.6 ± 2.0 c | 16.6 ± 2.0 a |
| 0.2 | 100 ± 0.0 c | 100 ± 0.0 c | 77.3 ± 4.6 c | 26.0 ± 5.2 c | 33.0 ± 5.2 c |
| 0.4 | 100 ± 0.0 c | 100 ± 0.0 c | 90.3 ± 1.1 c | 59.3 ± 6.6 c | 51.6 ± 17.6 c |
| 0.6 | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c |
| Fraction (mg/mL) | Blastula | Gastrula | Trochophore | Veliger | Hippo Stage |
| 0.0125 | 13.3 ± 3.5 | 4.0 ± 1.0 | 5.6 ± 3.5 | 1.3 ± 0.6 | 2.0 ± 1.0 |
| 0.025 | 21.3 ± 2.5 c | 33.0 ± 4.5 c | 7.6 ± 2.3 | 11.3 ± 2.5 | 3.6 ± 2.5 |
| 0.05 | 37.6 ± 1.5 c | 57.6 ± 4.1 c | 24.3 ± 3.7 c | 29.3 ± 7.2 c | 24.3 ± 6.0 c |
| 0.1 | 65.3 ± 9.0 c | 88.6 ± 2.0 c | 42.6 ± 3.8 c | 51.3 ± 5.5 c | 51.0 ± 9.1 c |
| 0.2 | 100 ± 0.0 c | 100 ± 0.0 c | 64.0 ± 2.6 c | 67.7 ± 4.2 c | 57.3 ± 4.7 c |
| 0.4 | 100 ± 0.0 c | 100 ± 0.0 c | 95.7 ± 2.5 c | 92.7 ± 3.0 c | 75.3 ± 4.5 c |
| 0.6 | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 c |
| Lethal Concentrations (LC) (mg/mL) | |||
|---|---|---|---|
| Saline Extract | LC10 | LC50 | LC90 |
| Blastula | 0.0056 (0.0046–0.0067) | 0.0420 (0.040–0.0434) | 0.1552 (0.1538–0.1567) |
| Gastrula | 0.0065 (0.0054–0.0077) | 0.0417 (0.0405–0.0428) | 0.1613 (0.1601–0.1624) |
| Trochophore | 0.0166 (0.0150–0.0183) | 0.0897 (0.0880–0.0913) | 0.3981 (0.3964–0.3997) |
| Veliger | 0.0312 (0.0219–0.0405) | 0.3734 (0.3641–0.3827) | 0.573 (0.5636–0.5823) |
| Hippo Stage | 0.0476 (0.0371–0.0580) | 0.3970 (0.3866–0.4075) | 0.5925 (0.582–0.6029) |
| Adult snails (24 h) | 3.73 (3.15–4.30) | 6.6 (6.02–7.18) | 9.47 (8.898–10.05) |
| Fraction | LC10 | LC50 | LC90 |
| Blastula | 0.0073 (0.005–0.0096) | 0.0478 (0.0455–0.0501) | 0.1681 (0.1658–0.1703) |
| Gastrula | 0.0071 (0.006–0.0082) | 0.0419 (0.0408–0.0430) | 0.1579 (0.1567–0.1590) |
| Trochophore | 0.0227 (0.019–0.0263) | 0.1582 (0.1544–0.1620) | 0.3791 (0.3753–0.3829) |
| Veliger | 0.016 (0.0126–0.0194) | 0.0974 (0.094–0.1008) | 0.3685 (0.3651–0.3719) |
| Hippo Stage | 0.0178 (0.0143–0.0213) | 0.0970 (0.0935–0.1005) | 0.5489 (0.5454–0.5524) |
| Adult Snails (24 h) | 0.37 (0.26–0.49) | 0.87 (0.75–0.99) | 1.70 (1.58–1.82) |
| Experimental Group (mg/mL) | Exposure Time (Minutes) | ||||
|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | |
| Controls | |||||
| Negative Control H2O | 3 | 3 | 3 | 3 | 3 |
| Niclosamide 0.001 | 0 | 0 | 0 | 0 | 0 |
| B. monandra Saline extract | |||||
| 4.0 | 3 | 2 | 1 | 1 | 1 |
| 3.0 | 3 | 2 | 1 | 1 | 1 |
| 2.0 | 3 | 3 | 3 | 3 | 3 |
| 1.0 | 3 | 3 | 3 | 3 | 3 |
| 0.5 | 3 | 3 | 3 | 3 | 3 |
| B. monandra Fraction | |||||
| 2.0 | 3 | 2 | 1 | 0 | 0 |
| 1.0 | 3 | 2 | 2 | 1 | 1 |
| 0.75 | 3 | 3 | 3 | 2 | 1 |
| 0.5 | 3 | 3 | 3 | 3 | 3 |
| Metabolite Class | System | Reference Compounds | Developer |
|---|---|---|---|
| Cinnamic derivatives | (90:5:5) | Caffeic acid | AlCl3 |
| Flavonoids | (90:5:5) | Quercetin | AlCl3 |
| Hydrolysable tannins | (90:5:5) | Gallic acid | FeCl3 |
| Condensed Tannins | (90:5:5) | Catechin | Vanillin Hydrochloric + Δ |
| Coumarin | (50:50:50) | Coumarin | KOH |
| Terpenes/Steroids | (90:10) | β-sitosterol | Liebermann-Burchard + Δ |
| Saponins | (16:10:2.5) | Escin | Liebermann-Burchard + Δ |
| Anthracenes | (20:30:15:0.5) | Sennoside B | HNO3 + Δ + KOH |
| Sugars | (100:11:11:26) | Glucose | Thymol + H2SO4 + Δ |
| Alkaloids | (70:20:10) | Piperine | Dragendorff |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, T.W.d.A.; Batista, J.J.; Ferreira, S.A.d.O.; Sampaio, M.d.V.L.; Pereira, D.R.; Ferreira, M.R.A.; Soares, L.A.L.; Melo, A.M.M.d.A.; Albuquerque, M.C.P.d.A.; Aires, A.d.L.; et al. Effect of Bauhinia monandra Kurz Leaf Preparations on Embryonic Stages and Adult Snails of Biomphalaria glabrata (Say, 1818), Schistosoma mansoni Cercariae and Toxicity in Artemia salina. Molecules 2022, 27, 4993. https://doi.org/10.3390/molecules27154993
Aguiar TWdA, Batista JJ, Ferreira SAdO, Sampaio MdVL, Pereira DR, Ferreira MRA, Soares LAL, Melo AMMdA, Albuquerque MCPdA, Aires AdL, et al. Effect of Bauhinia monandra Kurz Leaf Preparations on Embryonic Stages and Adult Snails of Biomphalaria glabrata (Say, 1818), Schistosoma mansoni Cercariae and Toxicity in Artemia salina. Molecules. 2022; 27(15):4993. https://doi.org/10.3390/molecules27154993
Chicago/Turabian StyleAguiar, Thierry Wesley de Albuquerque, José Josenildo Batista, Silvio Assis de Oliveira Ferreira, Maíra de Vasconcelos Lima Sampaio, Dewson Rocha Pereira, Magda Rhayanny Assunção Ferreira, Luiz Alberto Lira Soares, Ana Maria Mendonça de Albuquerque Melo, Mônica Camelo Pessoa de Azevedo Albuquerque, André de Lima Aires, and et al. 2022. "Effect of Bauhinia monandra Kurz Leaf Preparations on Embryonic Stages and Adult Snails of Biomphalaria glabrata (Say, 1818), Schistosoma mansoni Cercariae and Toxicity in Artemia salina" Molecules 27, no. 15: 4993. https://doi.org/10.3390/molecules27154993
APA StyleAguiar, T. W. d. A., Batista, J. J., Ferreira, S. A. d. O., Sampaio, M. d. V. L., Pereira, D. R., Ferreira, M. R. A., Soares, L. A. L., Melo, A. M. M. d. A., Albuquerque, M. C. P. d. A., Aires, A. d. L., Araújo, H. D. A. d., & Coelho, L. C. B. B. (2022). Effect of Bauhinia monandra Kurz Leaf Preparations on Embryonic Stages and Adult Snails of Biomphalaria glabrata (Say, 1818), Schistosoma mansoni Cercariae and Toxicity in Artemia salina. Molecules, 27(15), 4993. https://doi.org/10.3390/molecules27154993