The Current Situation of Pea Protein and Its Application in the Food Industry
Abstract
:1. Introduction
2. Extraction Process of Pea Protein from Pea
2.1. Wet Extraction: Alkali Extraction/Isoelectric Precipitation
2.2. Dry Fractionation: Air Classification and Size Reduction
2.3. Salt Extraction and Micellization
2.4. Mild Fractionation
3. Chemistry and Molecular Structure
4. Modification Techniques to Improve Functional Properties
4.1. Physical Modification
4.1.1. Heat Treatment
4.1.2. High-Pressure Treatment
4.1.3. Heat with Shear Treatment (Extrusion)
4.1.4. Cold Atmospheric Pressure Plasma Treatment
4.1.5. Ultrasonic Treatment
4.2. Chemical Modification
4.2.1. Glycation
4.2.2. Acylation (Acetylation and Succinylation)
4.2.3. Deamidation
4.3. Biological Modification
4.3.1. Fermentation
4.3.2. Enzymatic Modification
5. Techno Functional Properties
5.1. Solubility
5.2. Water Holding Capacity
5.3. Oil Holding Capacity
5.4. Emulsion Ability
5.5. Gelation
5.6. Foaming Properties
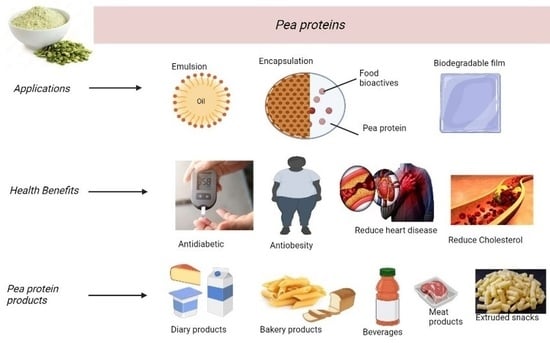
6. Pea Protein Application and Its Health Benefits
6.1. Food Emulsifier
6.2. Encapsulation Techniques for Bioactive Ingredients
6.3. Pea Protein-Based in Films
6.4. Health Attributes of Pea Proteins
6.5. Commercially Available Pea Protein Products
6.5.1. Cereal and Bakery Products
Bread
Pasta
6.5.2. Extruded Snacks
6.5.3. Beverages
6.5.4. Dairy Products
6.5.5. Meat Products
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balandran-Quintana, R.R.; Mendoza-Wilson, A.M.; Montfort, G.R.C.; Huerta-Ocampo, J.A. Plant-based proteins. In Proteins: Sustainable Source, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 97–130. [Google Scholar]
- Kumar, M.; Tomar, M.; Potkule, J.; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Pradhan, P.C.; Bhushan, B.; Anitha, T.; et al. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Khan, T.N.; Meldrum, A.; Croser, J.S. Pea: Overview; Elsevier: Melbourne, Australia, 2016; Volume 1, pp. 324–333. [Google Scholar]
- Bajaj, P.R.; Tang, J.; Sablani, S.S. Pea protein isolates: Novel wall materials for microencapsulating flaxseed oil. Food Bioprocess Technol. 2015, 8, 2418–2428. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Asavajaru, P.; Lam, A. Pea: A sustainable vegetable protein crop. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 145–164. [Google Scholar]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential health benefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Liu, P.; Wu, J. Identification of angiotensin converting enzyme 2 (ACE2) up-regulating peptides from pea protein hydrolysate. J. Funct. Foods 2019, 60, 103395. [Google Scholar] [CrossRef]
- Sun, W.; Xiong, Y.L. Stabilization of cooked cured beef color by radical-scavenging pea protein and its hydrolysate. LWT-Food Sci. Technol. 2015, 61, 352–358. [Google Scholar] [CrossRef]
- Aluko, R.E.; Girgih, A.T.; He, R.; Malomo, S.; Li, H.; Offengenden, M.; Wu, J. Structural and functional characterization of yellow field pea seed (Pisum sativum L.) protein-derived antihypertensive peptides. Food Res. Int. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Krumreich, F.D.; Zandoná, G.P.; Gularte, M.A.; Borges, C.D.; Zambiazi, R.C. Production of propolis extract microparticles with concentrated pea protein for application in food. Food Bioprocess Technol. 2019, 12, 729–740. [Google Scholar] [CrossRef]
- Philipp, C.; Emin, M.A.; Buckow, R.; Silcock, P.; Oey, I. Pea protein-fortified extruded snacks: Linking melt viscosity and glass transition temperature with expansion behaviour. J. Food Eng. 2018, 217, 93–100. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Panouille, M.; Huc-Mathis, D.; Moulin, G.; Saint-Eve, A.; Irlinger, F.; Bonnarme, P.; Michon, C.; Souchon, I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Markedal, K.E.; Petersen, I.L.; Sorensen, J.C.; Kuktaite, R. The impact of newly produced protein and dietary fiber rich fractions of yellow pea (Pisum sativum L.) on the structure and mechanical properties of pasta-like sheets. Food Res. Int. 2018, 106, 607–618. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-based delivery systems for the nanoencapsulation of food ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, T.N.; Parveen, R.; Fatima, S. Characterization, biomedical and agricultural applications of protease inhibitors: A review. Int. J. Biol. Macromol. 2016, 91, 1120–1133. [Google Scholar] [CrossRef]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Cabrilo, S.B. Techno-functional properties of pea (Pisum sativum) protein isolates: A review. Acta Period. Technol. 2015, 46, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Reinkensmeier, A.; Bußler, S.; Schlüter, O.; Rohn, S.; Rawel, H.M. Characterization of individual proteins in pea protein isolates and air classified samples. Food Res. Int. 2015, 76, 160–167. [Google Scholar] [CrossRef]
- Adenekan, M.K.; Fadimu, G.J.; Odunmbaku, L.A.; Oke, E.K. Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci. Nutr. 2018, 6, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Rempel, C.; Geng, X.; Zhang, Y. Industrial-scale preparation of pea flour fractions with enhanced nutritive composition by dry fractionation. Food Chem. 2019, 276, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re) formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Do Carmo, C.S.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjolen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein-and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- Feyzi, S.; Milani, E.; Golimovahhed, Q.A. Grass pea (Lathyrus sativus L.) protein isolate: The effect of extraction optimization and drying methods on the structure and functional properties. Food Hydrocoll. 2018, 74, 187–196. [Google Scholar] [CrossRef]
- Qiaoyun, C.; Xinghong, N.; Liang, Z.; Zheng, T.; Jin, L.; Kang, S.; Xuan, C.; Xinghui, L. Optimization of protein extraction and decoloration conditions for tea residues. Hortic. Plant J. 2017, 3, 172–176. [Google Scholar]
- Del Mar Contreras, M.; Lama-Munoz, A.; Gutierrez-Perez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Guckeisen, T.; Hosseinpour, S.; Peukert, W. Effect of pH and urea on the proteins secondary structure at the water/air interface and in solution. J. Colloid Interface Sci. 2021, 590, 38–49. [Google Scholar] [CrossRef]
- Zhang, C.; Sanders, J.P.; Xiao, T.T.; Bruins, M.E. How does alkali aid protein extraction in green tea leaf residue: A basis for integrated biorefinery of leaves. PLoS ONE 2015, 10, e0133046. [Google Scholar]
- Dhaliwal, S.K.; Salaria, P.; Kaushik, P. Pea seed proteins: A nutritional and nutraceutical update. In Grain and Seed Proteins Functionality; Intech Open: London, UK, 2021. [Google Scholar]
- Maity, T.; Saxena, A. Recent Advances in Processing of Peas. Veg. Process. Bioact. Compd. 2017, 396–424. [Google Scholar]
- Vogelsang-O’Dwyer, M.; Zannini, E.; Arendt, E.K. Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci. Technol. 2021, 110, 364–374. [Google Scholar] [CrossRef]
- Hadnadev, M.S.; Hadnadev-Dapčević, T.; Pojić, M.M.; Šarić, B.M.; Mišan, A.Č.; Jovanov, P.T.; Sakač, M.B. Progress in vegetable proteins isolation techniques: A review. Food Feed Res. 2017, 44, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Nik, M.; Boostani, S.; Jafari, S.M. Pea proteins as emerging biopolymers for the emulsification and encapsulation of food bioactives. Food Hydrocoll. 2021, 107474. [Google Scholar]
- Sozer, N.; Holopainen-Mantila, U.; Poutanen, K. Traditional and new food uses of pulses. Cereal Chem. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Sweers, L.J.H.; Politiek, R.G.A.; Lakemond, C.M.M.; Bruins, M.E.; Boom, R.M.; Fogliano, V.; Mishyna, M.; Keppler, J.K.; Schutyser, M.A.I. Dry fractionation for protein enrichment of animal by-products and insects: A review. J. Food Eng. 2022, 313, 110759. [Google Scholar] [CrossRef]
- Price, C.; Kiszonas, A.M.; Smith, B.; Morris, C.F. Roller milling performance of dry yellow split peas: Mill stream composition and functional characteristics. Cereal Chem. 2021, 98, 462–473. [Google Scholar] [CrossRef]
- Jones, J.M.; Adams, J.; Harriman, C.; Miller, C.; Van der Kamp, J.W. Nutritional impacts of different whole grain milling techniques: A review of milling practices and existing data. Cereal Foods World 2015, 60, 130–139. [Google Scholar] [CrossRef]
- Assatory, A.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Dry fractionation methods for plant protein, starch and fiber enrichment: A review. Trends Food Sci. Technol. 2019, 86, 340–351. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sorensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A. Charging and separation behavior of gluten–starch mixtures assessed with a custom-built electrostatic separator. Sep. Purif. Technol. 2015, 152, 164–171. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Jafari, M.; Rajabzadeh, A.R.; Legge, R.L. Solvent-free production of protein-enriched fractions from navy bean flour using a triboelectrification-based approach. J. Food Eng. 2016, 174, 21–28. [Google Scholar] [CrossRef]
- Vitelli, M.; Rajabzadeh, A.R.; Tabtabaei, S.; Assatory, A.; Shahnam, E.; Legge, R.L. Effect of hammer and pin milling on triboelectrostatic separation of legume flour. Powder Technol. 2020, 372, 317–324. [Google Scholar] [CrossRef]
- Klupsaite, D.; Juodeikiene, G. Legume: Composition, protein extraction and functional properties. A review. Chem. Technol. 2015, 66, 5–12. [Google Scholar] [CrossRef]
- Muranyi, I.S.; Otto, C.; Pickardt, C.; Osen, R.; Koehler, P.; Schweiggert-Weisz, U. Influence of the isolation method on the techno functional properties of protein isolates from Lupinus angustifolius L. J. Food Sci. 2016, 81, C2656–C2663. [Google Scholar] [CrossRef]
- Geerts, M.; Mienis, E.; Nikiforidis, C.V.; van der Padt, A.; van der Goot, A.J. Mildly refined fractions of yellow peas show rich behaviour in thickened oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2017, 41, 251–258. [Google Scholar] [CrossRef]
- Kornet, C.; Venema, P.; Nijsse, J.; van der Linden, E.; van der Goot, A.J.; Meinders, M. Yellow pea aqueous fractionation increases the specific volume fraction and viscosity of its dispersions. Food Hydrocoll. 2020, 99, 105332. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, T.Z.; Chigwedere, C.M.; Ai, Y. A current review of structure, functional properties, and industrial applications of pulse starches for value-added utilization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3061–3092. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Avila Ruiz, G.; Arts, A.; Minor, M.; Schutyser, M. A hybrid dry and aqueous fractionation method to obtain protein-rich fractions from quinoa (Chenopodium quinoa willd). Food Bioprocess Technol. 2016, 9, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Functional analysis of mildly refined fractions from yellow pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Berghout, J.A.M.; Pelgrom, P.J.M.; Schutyser, M.A.I.; Boom, R.M.; Van Der Goot, A.J. Sustainability assessment of oilseed fractionation processes: A case study on lupin seeds. J. Food Eng. 2015, 150, 117–124. [Google Scholar] [CrossRef]
- Carvajal-Pinero, J.M.; Ramos, M.; Jim enez-Rosado, M.; Perez-Puyana, V.; Romero, A. Development of pea protein bioplastics by a thermomoulding process: Effect of the mixing stage. J. Polym. Environ. 2019, 27, 968–978. [Google Scholar] [CrossRef]
- Acquah, C.; Zhang, Y.; Dube, M.A.; Udenigwe, C.C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Bivolarevic, V. Comparative study of the functional properties of three legume seed isolates: Adzuki, pea and soy bean. J. Food Sci. Technol. 2015, 52, 2779–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein-polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.A.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Babault, N.; Païzis, C.; Deley, G.; Guérin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, Placebo-controlled clinical trial vs. Whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The effects of whey vs. pea protein on physical adaptations following 8-weeks of high-intensity functional training (HIFT): A pilot study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: A double-blind, cross-over trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol. Biotechnol. 2018, 56, 257. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R. Functional and engineered colloids from edible materials for emerging applications in designing the food of the future. Adv. Funct. Mater. 2018, 30, 1806809. [Google Scholar] [CrossRef]
- Robinson, G.H.; Domoney, C. Perspectives on the genetic improvement of health-and nutrition-related traits in pea. Plant Physiol. Biochem. 2021, 158, 353–362. [Google Scholar] [CrossRef]
- Antonets, K.S.; Belousov, M.V.; Sulatskaya, A.I.; Belousova, M.E.; Kosolapova, A.O.; Sulatsky, M.I.; Andreeva, E.A.; Zykin, P.A.; Malovichko, Y.V.; Shtark, O.Y.; et al. Accumulation of storage proteins in plant seeds is mediated by amyloid formation. PLoS Biol. 2020, 18, e3000564. [Google Scholar]
- Djoullah, A.; Husson, F.; Saurel, R. Gelation behaviors of denaturated pea albumin and globulin fractions during transglutaminase treatment. Food Hydrocoll. 2018, 77, 636–645. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Functionalisation of pea protein by tryptic hydrolysis—characterisation of interfacial and functional properties. Food Hydrocoll. 2019, 86, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse proteins: From processing to structure-function relationships. In Grain Legumes; Intech: London, UK, 2016; p. 55. [Google Scholar]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar]
- Kreplak, J.; Madoui, M.A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Daba, S.D.; Morris, C.F. Pea proteins: Variation, composition, genetics, and functional properties. Cereal Chem. 2022, 99, 8–20. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Bao Chau, N.H.; Trivedi, J.; Dissanayake, M.; Vasiljevic, T. Impact of selected process parameters on solubility and heat stability of pea protein isolate. Lebensm.-Wiss.-Technol.-Food Sci. Technol. 2019, 102, 246–253. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of processing on the chemistry and functionality of food proteins. In Proteins in Food Processing; Woodhead Publishing: Philadelphia, PA, USA, 2018; pp. 27–45. [Google Scholar]
- Chao, D.F.; Jung, S.; Aluko, R.E. Physicochemical and functional properties of high pressure-treated isolated pea protein. Innov. Food Sci. Emerg. Technol. 2018, 45, 179–185. [Google Scholar] [CrossRef]
- Peng, W.W.; Kong, X.Z.; Chen, Y.M.; Zhang, C.M.; Yang, Y.X.; Hua, Y.F. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll. 2016, 52, 301–310. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Wu, J.; Ayun, Q.; Van der Meeren, P. Maillard conjugation as an approach to improve whey proteins functionality: A review of conventional and novel preparation techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Aliabadi, S.S.; Hosseini, S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef]
- Queiros, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yildiz, G.; dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016, 55, 200–209. [Google Scholar] [CrossRef]
- Do Carmo, C.S.; Nunes, A.N.; Silva, I.; Maia, C.; Poejo, J.; Ferreira-Dias, S.; Nogueira, I.; Bronze, R.; Duarte, C.M.M. Formulation of pea protein for increased satiety and improved foaming properties. RSC Adv. 2016, 6, 6048–6057. [Google Scholar] [CrossRef]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Oey, I.; Roohinejad, S. Effect of extrusion on the anti-nutritional factors of food products: An overview. Food Control 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlstrom, C.; Ostbring, K.; Rayner, M.; Purhagen, J.K. Development of high-moisture meat analogues with hemp and soy protein using extrusion cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2018, 59, 2506–2533. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecular weight distribution and secondary structure as determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Bussler, S.; Steins, V.; Ehlbeck, J.; Schluter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent advances in the application of cold plasma technology in foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of dielectric barrier discharge (DBD) cold plasma treatment on physicochemical and functional properties of peanut protein. Food Bioprocess Technol. 2018, 11, 344–354. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Ye, Q.Y.; Biviano, M.; Mettu, S.; Zhou, M.F.; Dagastine, R.; Ashokkumar, M. Modification of pea protein isolate for ultrasonic encapsulation of functional liquids. RSC Adv. 2016, 6, 106130–106140. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high-intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Ertugrul, U.; Namli, S.; Tas, O.; Kocadagli, T.; Gokmen, V.; Sumnu, S.G.; Oztop, M.H. Pea protein properties are altered following glycation by microwave heating. LWT 2021, 150, 111939. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Goli, S.A.H.; Nasirpour, A. Evaluation of biopolymer-based emulsion for delivering conjugated linoleic acid (CLA) as a functional ingredient in beverages. J. Dispers. Sci. Technol. 2015, 36, 778–788. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Goli, S.A.H.; Doost, A.S.; Dewettinck, K.; Van der Meeren, P. Bioparticles of flaxseed protein and mucilage enhance the physical and oxidative stability of flaxseed oil emulsions as a potential natural alternative for synthetic surfactants. Colloids Surf. B Biointerfaces 2019, 184, 110489. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Whey protein-carbohydrate conjugates. In Whey Proteins; Academic Press: Cambridge, MA, USA, 2019; pp. 249–280. [Google Scholar]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B Biointerfaces 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Zha, F.; Gao, K.; Rao, J.; Chen, B. Maillard-driven chemistry to tune the functionality of pea protein: Structure characterization, site-specificity, and aromatic profile. Trends Food Sci. Technol. 2021, 114, 658–671. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Leza, G.L.; Martínez, L.M.; Chuck-Hernandez, C. Impact of Hydrolysis, Acetylation or Succinylation on Functional Properties of Plant-Based Proteins: Patents, Regulations, and Future Trends. Processes 2022, 10, 283. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Tomar, S.K. Effect of succinylation on physicochemical and functional properties of milk protein concentrate. Food Res. Int. 2015, 72, 223–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, N.; Li, Y.; Cheng, S.; Jiang, C.; Lin, S. Effects of electron beam irradiation (EBI) on structure characteristics and thermal properties of walnut protein flour. Food Res. Int. 2017, 100, 850–857. [Google Scholar] [CrossRef]
- Shah, N.N.; Umesh, K.V.; Singhal, R.S. Hydrophobically modified pea proteins: Synthesis, characterization and evaluation as emulsifiers in eggless cake. J. Food Eng. 2019, 255, 15–23. [Google Scholar] [CrossRef]
- Kumagai, H.; Urade, R. Deamidation of gluten proteins as a tool for improving the properties of bread. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2019; pp. 3–11. [Google Scholar]
- He, W.; Yang, R.; Zhao, W. Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 2019, 277, 655–663. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Zhang, X.; Zhang, G.; Zhou, J.; Chen, J. Application Prospect of Protein-Glutaminase in the Development of Plant-Based Protein Foods. Foods 2022, 11, 440. [Google Scholar] [CrossRef]
- Fang, L.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Lin, J. Enhancing the usability of pea protein isolate in food applications through modifying its structural and sensory properties via deamidation by glutaminase. J. Agric. Food Chem. 2020, 68, 1691–1697. [Google Scholar] [CrossRef]
- Schlegel, K.; Leidigkeit, A.; Eisner, P.; Schweiggert-Weisz, U. Techno functional and sensory properties of fermented lupin protein isolates. Foods 2019, 8, 678. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S. Pulse protein ingredient modification. J. Sci. Food Agric. 2022, 102, 892–897. [Google Scholar] [CrossRef]
- Boroojeni, F.G.; Kozłowski, K.; Jankowski, J.; Senz, M.; Wisniewska, M.; Boros, D.; Drazbo, A.; Zentek, J. Fermentation and enzymatic treatment of pea for turkey nutrition. Anim. Feed Sci. Technol. 2018, 237, 78–88. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Huang, G.Q.; Xu, T.C.; Liu, L.N.; Xiao, J.X. Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Glusac, J.; Davidesko-Vardi, I.; Isaschar-Ovdat, S.; Kukavica, B.; Fishman, A. Gel-like emulsions stabilized by tyrosinasecrosslinked potato and zein proteins. Food Hydrocoll. 2018, 82, 53–63. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadifard, N.; Murueta, J.H.C.; Abedian-Kenari, A.; Motamedzadegan, A.; Jamali, H. Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with sds-page. J. Food Sci. Technol. 2016, 53, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.C.; Liu, N.; Feng, C.X. Research on the effect of papain co-extrusion on pea protein and enzymolysis antioxidant peptides. J. Food Process. Preserv. 2017, 41, e13301. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, Z.F.; Zhang, H.; Ma, S.; Kong, L. Structural characteristics and functional properties of walnut glutelin as hydrolyzed: Effect of enzymatic modification. Int. J. Food Prop. 2019, 22, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Garcia Arteaga, V.; Apestegui Guardia, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Jiang, S.S.; Ding, J.Z.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, M. Solubility of proteins. ADMET DMPK 2020, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Zhang, R.; Yao, P. Influence of pea protein aggregates on the structure and stability of pea protein/soybean polysaccharide complex emulsions. Molecules 2015, 20, 5165–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low- and high moisture extrusion of pulse proteins as plant-based meat ingredients: A review. Cereal Foods World 2020, 65, 12–14. [Google Scholar]
- Stone, A.K.; Avarmenko, N.A.; Warkentin, T.D.; Nickerson, M.T. Functional properties of protein isolates from different pea cultivars. Food Sci. Biotechnol. 2015, 24, 827–833. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. Succinylation of food proteins-a concise review. LWT 2022, 154, 112866. [Google Scholar] [CrossRef]
- Zhao, H.F.; Shen, C.; Wu, Z.J.; Zhang, Z.; Xu, C.M. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, e13157. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Zhang, Y.; Zhang, W. Influence of succinylation on physicochemical property of yak casein micelles. Food Chem. 2016, 190, 836–842. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.L.; Jezowski, P.; Singh, A.P. Pea protein for hempseed oil nanoemulsion stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Tang, C.H. Gel-like pea protein Pickering emulsions at pH3.0 as a potential intestine-targeted and sustained-release delivery system for β-carotene. Food Res. Int. 2016, 79, 64–72. [Google Scholar] [CrossRef]
- Jiang, S.; Yildiz, G.; Ding, J.; Andrade, J.; Rababahb, T.M.; Almajwalc, A.; Abulmeatyc, M.M.; Feng, H. Pea protein nanoemulsion and nanocomplex as carriers for protection of cholecalciferol (vitamin D3). Food Bioprocess Technol. 2019, 12, 1031–1040. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Foodgrade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Huang, Q. Recent advances on food-grade particles stabilized pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef]
- Velez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocoll. 2020, 105, 105775. [Google Scholar] [CrossRef]
- Tomé, A.S.; Pires, C.; Batista, I.; Sousa, I.; Raymundo, A. Protein gels and emulsions from mixtures of Cape hake and pea proteins. J. Sci. Food Agric. 2015, 95, 289–298. [Google Scholar] [CrossRef]
- Mession, J.L.; Roustel, S.; Saurel., R. Interactions in casein micelle—Pea protein system (part I): Heat-induced denaturation and aggregation. Food Hydrocoll. 2017, 67, 229–242. [Google Scholar] [CrossRef]
- Munialo, C.D.; van der Linden, E.; Akt, K.; de Jongh, H.H.J. Quantitative analysis of the network structure that underlines the transitioning in mechanical responses of pea protein gels. Food Hydrocoll. 2015, 49, 104–117. [Google Scholar] [CrossRef]
- Silva, J.V.C.; Balakrishnan, G.; Schmitt, C.; Chassenieux, C.; Nicolai, T. Heat-induced gelation of aqueous micellar casein suspensions as affected by globular protein addition. Food Hydrocoll. 2018, 82, 258–267. [Google Scholar] [CrossRef]
- Mession, J.L.; Roustel, S.; Saurel, R. Interactions in casein micelle—Pea protein system (part II): Mixture acid gelation with glucono-d-lactone. Food Hydrocoll. 2017, 73, 344–357. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Utilization of pulse protein-xanthan gum complexes for foam stabilization: The effect of protein concentrate and isolate at various pH. Food Chem. 2020, 316, 126282. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; Silva, J.V.; Saffon, M.; Dombrowski, J. On the foaming properties of plant proteins: Current status and future opportunities. Trends Food Sci. Technol. 2021, 118, 261–272. [Google Scholar] [CrossRef]
- O’sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Amid, B.T.; Kazemi, M.; Hedayatnia, S. Encapsulation properties, release behavior and physicochemical characteristics of water-in-oil-in-water (W/O/W) emulsion stabilized with pectin-pea protein isolate conjugate and Tween 80. Food Hydrocoll. 2016, 61, 599–608. [Google Scholar] [CrossRef]
- Jafari, S.M.; Doost, A.S.; Nasrabadi, M.N.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Amagliani, L.; Schmitt, C. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Nunes, J.C.; Lima, B.N.B.; Pedrosa, C.; Calado, V.; Torres, A.G.; Pierucci, A.P.T.R. Effective stabilization of CLA by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Karaca, A.C. Encapsulation of black pepper seed oil using maltodextrin and pea protein. Food Sci. Technol. Int. 2019, 26, 369–378. [Google Scholar] [CrossRef]
- Yildiz, G.; Ding, J.; Gaur, S.; Andrade, J.; Engeseth, N.E.; Feng, H. Microencapsulation of docosahexaenoic acid (DHA) with four wall materials including pea protein-modified starch complex. Int. J. Biol. Macromol. 2018, 114, 935–941. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Zieba, E.; Lisiecki, S.; Stadnik, J.; Baraniak, B. Microstructure and functional properties of sorbitol-plasticized pea protein isolate emulsion films: Effect of lipid type and concentration. Food Hydrocoll. 2016, 60, 353–363. [Google Scholar] [CrossRef]
- Huntrakul, K.; Yoksan, R.; Sane, A.; Harnkarnsujarit, N. Effects of pea protein on properties of cassava starch edible films produced by blown-film extrusion for oil packaging. Food Packag. Shelf Life 2020, 24, 100480. [Google Scholar] [CrossRef]
- Krefting, J. The appeal of pea protein. J. Ren. Nutr. 2017, 27, e31–e33. [Google Scholar] [CrossRef] [Green Version]
- Lefranc-Millot, C.; Teichman-Dubois, V. Protein from vegetable sources: A focus on pea protein. In Novel Proteins for Food, Pharmaceuticals and Agriculture; John Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 197–216. [Google Scholar]
- Stilling, K. Health Benefits of Pea Protein Isolate: A Comparative Review. SURG J. 2020, 1–10. [Google Scholar] [CrossRef]
- Bustillos, M.A.; Jonchere, C.; Garnier, C.; Reguerre, A.L.; Della Valle, G. Rheological and microstructural characterization of batters and sponge cakes fortified with pea proteins. Food Hydrocoll. 2020, 101, 105553. [Google Scholar] [CrossRef]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Sahagún, M.; Gomez, M. Assessing influence of protein source on characteristics of gluten-free breads optimising their hydration level. Food Bioprocess Technol. 2018, 11, 1686–1694. [Google Scholar] [CrossRef]
- Sahagún, M.; Benavent-Gil, Y.; Rosell, C.M.; Gómez, M. Modulation of in vitro digestibility and physical characteristics of protein enriched gluten free breads by defining hydration. LWT 2020, 117, 108642. [Google Scholar] [CrossRef]
- Linares-García, L.; Repo-Carrasco-Valencia, R.; Glorio Paulet, P.; Schoenlechner, R. Development of gluten-free and egg-free pasta based on quinoa (Chenopdium quinoa Willd) with addition of lupine flour, vegetable proteins and the oxidizing enzyme POx. Eur. Food Res. Technol. 2019, 245, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Wee, M.S.M.; Loud, D.E.; Tan, V.W.K.; Forde, C.G. Physical and sensory characterisation of noodles with added native and denatured pea protein isolate. Food Chem. 2019, 294, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Knoerzer, K.; Foerster, M.; Mayo, S.; Philipp, C.; Arcot, J. Low moisture extrusion of pea protein and pea fibre fortified rice starch blends. J. Food Eng. 2018, 231, 61–71. [Google Scholar] [CrossRef]
- Philipp, C.; Oey, I.; Silcock, P.; Beck, S.M.; Buckow, R. Impact of protein content on physical and microstructural properties of extruded rice starch-pea protein snacks. J. Food Eng. 2017, 212, 165–173. [Google Scholar] [CrossRef]
- Arbach, C.T.; Alves, I.A.; Serafini, M.R.; Stephani, R.; Perrone, I.T.; de Carvalho da Costa, J. Recent patent applications in beverages enriched with plant proteins. NPJ Sci. Food 2021, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, T.B.; Foegeding, E.A. Whey protein–pectin soluble complexes for beverage applications. Food Hydrocoll. 2017, 63, 130–138. [Google Scholar] [CrossRef]
- Trikusuma, M.; Paravisini, L.; Peterson, D.G. Identification of aroma compounds in pea protein UHT beverages. Food Chem. 2020, 312, 126082. [Google Scholar] [CrossRef]
- Baugreet, S.; Kerry, J.P.; Botineştean, C.; Allen, P.; Hamill, R.M. Development of novel fortified beef patties with added functional protein ingredients for the elderly. Meat Sci. 2016, 122, 40–47. [Google Scholar] [CrossRef]
- Baugreet, S.; Kerry, J.P.; Brodkorb, A.; Gomez, C.; Auty, M.; Allen, P.; Hamill, R.M. Optimisation of plant protein and transglutaminase content in novel beef restructured steaks for older adults by central composite design. Meat Sci. 2018, 142, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, A.; Sahar, A.; Sameen, A.; Saleem, A.; Tahir, A.T. Use of pea and rice protein isolates as source of meat extenders in the development of chicken nuggets. J. Food Process. Preserv. 2018, 42, e13763. [Google Scholar] [CrossRef]







| Extraction Method | Protein Yield (%) | Application | References |
|---|---|---|---|
| Alkali extraction/isoelectric precipitation | 62.6–80 | Improve texture and nutritional quality of food. Emulsifying material | [54] |
| Dry fractionation | 50–77 | Used encapsulating material | [55,56] |
| Salt extraction | 68.2–74.8 | Foaming capacity increases | [11,57] |
| Mild fractionation | 55–65 | Used to produce biodegradable natural polymer | [51,55,58] |
| Amino Acid | Pea Protein (g/100 g) | References |
|---|---|---|
| Essential amino acid | [63,64,65] | |
| Valine | 2.7–5 | |
| Leucine | 5.7–6.4 | |
| Isoleucine | 2.3–4.5 | |
| Methionine | 0.3–1.1 | |
| Phenylalanine | 3.7–5.5 | |
| Tryptophan | 0.7–1 | |
| Threonine | 2.5–3.9 | |
| Lysine | 4.7–5.7 | |
| Histidine | 1.6–2.5 | |
| Non-essential amino acid | [5,66,67] | |
| Alanine | 3.2–4.3 | |
| Aspartic acid | 8.9–11.5 | |
| Cystine | 0.2–1 | |
| Glutamic acid | 12.9–13.2 | |
| Glycine | 2.8–4.1 | |
| Proline | 3.1–4.5 | |
| Serine | 3.6–5.3 | |
| Tyrosine | 2.6–3.8 |
| Protein | Content | Solubility | Molecular Weight | Distinct Structural Features | Reference |
|---|---|---|---|---|---|
| Globulin | 65–80% | Salt solution | |||
| Legumin | 320–400 kDa | Hexameric protein with six subunits. Compact Quaternary structure. Has an acidic and basic polypeptide linked by disulfide bonds | [7,78] | ||
| Vicilin | 150–170 kDa | Trimeric protein. Combination of heterogenous polypeptides with no disulfide protein. Has hydrophilic surface more than legumin | [12,28] | ||
| Convicilin | 180–210 kDa | It can form trimers, including N-terminal with three convicilin molecules. Contain sulfur-containing amino acid. | [60] | ||
| Albumin | 10–20% | Water solution | 5–80 kDa | Two major fractions: a larger albumin protein comprising two polypeptides and a minor one. | [54] |
| PA1 | 5–9% | 10 kDa | Dimer | [28] | |
| PA2 | 10–20% | 50 kDa | Dimer | [12] | |
| Lectins | 2.5% | 50 kDa | Tetramer | [16] | |
| Lipoxygenases | <1% | n/a | n/a | [54] | |
| Serine/trypsin protease inhibitors | <2% | 10–16 kDa | Monomer | [7] | |
| Prolamin | 4–5% | Alcohol solution | n/a | Present in a small amount. Has high glutamine and proline content. | [37] |
| Glutelin | 3–4% | Insoluble | n/a | Class of prolamin-like protein. Only soluble in dilute acid or bases. Rich in hydrophobic amino acids. | [79] |
| Physical Modification | Modified Characteristics | Reference |
|---|---|---|
| High-pressure treatment (HPP) | Structural changes, foaming stability, and emulsifying property enhanced | [82] |
| Heat with shear treatment (Extrusion) | Improve the texture of protein | [122] |
| Cold atmospheric pressure plasma treatment | Improve solubility, emulsifying ability, and water holding capacity | [99] |
| Ultrasonic treatment | Improve gelling properties and enhance solubility | [101,102] |
| Chemical Modification | ||
| Glycation | Helps to reduce beany flavor | [103,123] |
| Acylation | Helps to improve solubility, Emulsion stability, water holding capacity, and foaming properties. | [116] |
| Deamidation | Improve solubilityReduces unpleasant beany flavor, bitterness, and lumpiness | [80] |
| Biological Modification | ||
| Fermentation | Improves digestibility of protein | [13] |
| Enzymatic modification | Improves protein solubility, hydrophobicity, emulsifying and foaming properties | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. https://doi.org/10.3390/molecules27165354
Shanthakumar P, Klepacka J, Bains A, Chawla P, Dhull SB, Najda A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules. 2022; 27(16):5354. https://doi.org/10.3390/molecules27165354
Chicago/Turabian StyleShanthakumar, Parvathy, Joanna Klepacka, Aarti Bains, Prince Chawla, Sanju Bala Dhull, and Agnieszka Najda. 2022. "The Current Situation of Pea Protein and Its Application in the Food Industry" Molecules 27, no. 16: 5354. https://doi.org/10.3390/molecules27165354
APA StyleShanthakumar, P., Klepacka, J., Bains, A., Chawla, P., Dhull, S. B., & Najda, A. (2022). The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules, 27(16), 5354. https://doi.org/10.3390/molecules27165354