Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties
Abstract
:1. Introduction
2. Stability of Anticancer Drugs
3. Stability of Anticancer Prodrugs
4. Stability of Anticancer Monoclonal Antibody
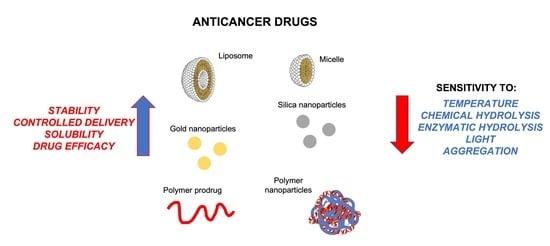
5. Anticancer Drugs in Nanoparticle Systems
5.1. Anticancer Prodrugs in Nanoparticles Systems
5.2. Combination Therapy in Nanoparticles Systems
5.3. Monoclonal Antibody in Nanoparticles Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- GLOBOCAN 2020: New Global Cancer Data|UICC. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 27 July 2022).
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Dosio, F.; Stella, B.; Cattel, L. Anticancer prodrugs: An overview of major strategies and recent developments. Curr. Top. Med. Chem. 2011, 11, 2346–2381. [Google Scholar] [CrossRef]
- Nasibullin, I.; Smirnov, I.; Ahmadi, P.; Vong, K.; Kurbangalieva, A.; Tanaka, K. Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Palombo, M.; Sinko, P. Recent trends in targeted anticancer prodrug and conjugate design. Curr. Med. Chem. 2008, 15, 1802–1826. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012; Bookshelf ID: NBK547852.
- Hawwa, A.F.; Millership, J.S.; Collier, P.S.; Vandenbroeck, K.; McCarthy, A.; Dempsey, S.; Cairns, C.; Collins, J.; Rodgers, C.; McElnay, J.C. Pharmacogenomic studies of the anticancer and immunosuppressive thiopurines mercaptopurine and azathioprine. Br. J. Clin. Pharmacol. 2008, 66, 517. [Google Scholar] [CrossRef] [PubMed]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs 2018, 32, 785–796. [Google Scholar] [CrossRef]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.M.; Wang, H.W.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.; et al. Randomized Phase II Study of First-Line Cladribine With Concurrent or Delayed Rituximab in Patients With Hairy Cell Leukemia. J. Clin. Oncol. 2020, 38, 1527–1538. [Google Scholar] [CrossRef]
- Johnson, S.A. Clinical pharmacokinetics of nucleoside analogues: Focus on haematological malignancies. Clin. Pharmacokinet. 2000, 39, 5–26. [Google Scholar] [CrossRef]
- Liao, J.; Peng, H.; Wei, X.; Song, Y.; Liu, C.; Li, D.; Yin, Y.; Xiong, X.; Zheng, H.; Wang, Q. A bio-responsive 6-mercaptopurine/doxorubicin based “Click Chemistry” polymeric prodrug for cancer therapy. Mater. Sci. Eng. C 2020, 108, 110461. [Google Scholar] [CrossRef]
- Mohammed, M.O.; Alkubaisi, H.M.M.; Haj, N.Q. A new prodrug and bioactivity evaluation of methotrexate based on Chitosan. Heliyon 2020, 6, e04223. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, B.; Jockusch, S.; Crespo-Hernández, C.E. Excited-State Dynamics of the Thiopurine Prodrug 6-Thioguanine: Can N9-Glycosylation Affect Its Phototoxic Activity? Molecules 2017, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Munshi, P.N.; Lubin, M.; Bertino, J.R. 6-thioguanine: A drug with unrealized potential for cancer therapy. Oncologist 2014, 19, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Power, D.G.; Kemeny, N.E. The role of floxuridine in metastatic liver disease. Mol. Cancer Ther. 2009, 8, 1015–1025. [Google Scholar] [CrossRef]
- Priest, D.G.; Schmitz, J.C.; Walle, T. Leucovorin as a prodrug. Adv. Exp. Med. Biol. 1993, 339, 31–40. [Google Scholar] [CrossRef]
- Van der Beek, J.N.; Oosterom, N.; Pieters, R.; de Jonge, R.; van den Heuvel-Eibrink, M.M.; Heil, S.G. The effect of leucovorin rescue therapy on methotrexate-induced oral mucositis in the treatment of paediatric ALL: A systematic review. Crit. Rev. Oncol. Hematol. 2019, 142, 1–8. [Google Scholar] [CrossRef]
- Buggia, I.; Locatelli, F.; Regazzi, M.B.; Zecca, M. Busulfan. Ann. Pharmacother. 1994, 28, 1055–1062. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Dai, R.Y.; Chen, Z.; Zhang, Y.H.; He, X.Z.; Zhou, J. Efficacy and safety of carmustine wafers in the treatment of glioblastoma multiforme: A systematic review. Turk. Neurosurg. 2014, 24, 639–645. [Google Scholar] [CrossRef]
- Ponticelli, C.; Escoli, R.; Moroni, G. Does cyclophosphamide still play a role in glomerular diseases? Autoimmun. Rev. 2018, 17, 1022–1027. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Breithaupt, H.; Dammann, A.; Aigner, K. Pharmacokinetics of dacarbazine (DTIC) and its metabolite 5-aminoimidazole-4-carboxamide (AIC) following different dose schedules. Cancer Chemother. Pharmacol. 1982, 9, 103–109. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, P.A.V.; Campelo Lopes, I.; Silva, E.H.C.; Bruzaca, E.E.S.; Alves, H.J.; Lima, M.I.S.; Tanaka, A.A. Electrochemical behaviour of anticancer drug lomustine and in situ evaluation of its interaction with DNA. J. Pharm. Biomed. Anal. 2019, 176, 112786. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saleem, T.J.; Stonesifer, C.J.; Khaleel, A.E.; Geskin, L.J. Management of Mycosis Fungoides with Topical Chlormethine/Mechlorethamine Gel: A Columbia University Cutaneous Lymphoma Center Experience. Acta Derm. Venereol. 2021, 101, adv00544. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Melphalan Flufenamide (Melflufen): First Approval. Drugs 2021, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.; Murray, G. Tumour cytochrome P450 and drug activation. Curr. Pharm. Des. 2002, 8, 1335–1347. [Google Scholar] [CrossRef]
- Mauz-Körholz, C.; Hasenclever, D.; Dörffel, W.; Ruschke, K.; Pelz, T.; Voigt, A.; Stiefel, M.; Winkler, M.; Vilser, C.; Dieckmann, K.; et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: The GPOH-HD-2002 study. J. Clin. Oncol. 2010, 28, 3680–3686. [Google Scholar] [CrossRef]
- Przepiorka, D.; Madden, T.; Ippoliti, C.; Estrov, Z.; Dimopoulos, M. Dosing of thioTEPA for myeloablative therapy. Cancer Chemother. Pharmacol. 1995, 37, 155–160. [Google Scholar] [CrossRef]
- Maanen, M.; Smeets, C.; Beijnen, J. Chemistry, pharmacology and pharmacokinetics of N,N’,N”-triethylenethiophosphoramide (ThioTEPA). Cancer Treat. Rev. 2000, 26, 257–268. [Google Scholar] [CrossRef]
- Agarwal, S.; Chadha, D.; Mehrotra, R. Molecular modeling and spectroscopic studies of semustine binding with DNA and its comparison with lomustine-DNA adduct formation. J. Biomol. Struct. Dyn. 2015, 33, 1653–1668. [Google Scholar] [CrossRef]
- Aubel-Sadron, G.; Londos-Gagliardi, D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie 1984, 66, 333–352. [Google Scholar] [CrossRef]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Fields, S.M.; Koeller, J.M. Idarubicin: A second-generation anthracycline. DICP 1991, 25, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Evison, B.J.; Sleebs, B.E.; Watson, K.G.; Phillips, D.R.; Cutts, S.M. Mitoxantrone, More than Just Another Topoisomerase II Poison. Med. Res. Rev. 2016, 36, 248–299. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.; Atrakchi, D.; Walker, M.D.; Horovitz, A.; Fridkin, M.; Shechter, Y. Converting bleomycin into a prodrug that undergoes spontaneous reactivation under physiological conditions. Toxicol. Appl. Pharmacol. 2019, 384, 114782. [Google Scholar] [CrossRef]
- Humeau, J.; Sauvat, A.; Cerrato, G.; Xie, W.; Loos, F.; Iannantuoni, F.; Bezu, L.; Lévesque, S.; Paillet, J.; Pol, J.; et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol. Med. 2020, 12, e11622. [Google Scholar] [CrossRef] [PubMed]
- Schnall, S.; Macdonald, J.S. Mitomycin therapy in gastric cancer. Oncology 1993, 50 (Suppl. 1), 70–77. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Torkelson, J.L. Long-term follow-up of stage III testicular carcinoma treated with mithramycin (plicamycin). Med. Pediatr. Oncol. 1995, 24, 327–328. [Google Scholar] [CrossRef]
- Fleming, R.A.; Miller, A.A.; Stewart, C.F. Etoposide: An update. Clin. Pharm. 1989, 8, 274–293. [Google Scholar]
- Muggia, F.M.; Kelley, S.L. Teniposide in adult solid tumors: A historical perspective. Semin. Oncol. 1992, 19, 43–50. [Google Scholar]
- Pobel, C.; Auclin, E.; Procureur, A.; Clément-Zhao, A.; Simonaggio, A.; Delanoy, N.; Vano, Y.A.; Thibault, C.; Oudard, S. Cabazitaxel schedules in metastatic castration-resistant prostate cancer: A review. Future Oncol. 2021, 17, 91–102. [Google Scholar] [CrossRef]
- Barata, P.C.; Sartor, A.O. Metastatic castration-sensitive prostate cancer: Abiraterone, docetaxel, or…. Cancer 2019, 125, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Dorrell, C.; Al-Fatease, A.; Allen-Petersen, B.L.; Woo, Y.; Bortnyak, Y.; Gheewala, R.; Sheppard, B.C.; Sears, R.C.; Alani, A.W.G. Microfluidics Formulated Liposomes of Hypoxia Activated Prodrug for Treatment of Pancreatic Cancer. Pharmaceutics 2022, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Tehrani, E.; Vafadar, A.; Keshavarzi, M.; Firouzabadi, N. Anticancer properties of vincristine is modulated by microRNAs in acute lymphoblastic leukemia Nalm6 cell line. Anticancer. Drugs 2022, 33, e680–e685. [Google Scholar] [CrossRef]
- Levêque, D.; Jehl, F. Clinical pharmacokinetics of vinorelbine. Clin. Pharmacokinet. 1996, 31, 184–197. [Google Scholar] [CrossRef]
- de Man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef]
- Ackermann, S.; Beckmann, M.W.; Thiel, F.; Bogenrieder, T. Topotecan in cervical cancer. Int. J. Gynecol. Cancer 2007, 17, 1215–1223. [Google Scholar] [CrossRef]
- Song, H.; Quan, F.; Yu, Z.; Zheng, M.; Ma, Y.; Xiao, H.; Ding, F. Carboplatin prodrug conjugated Fe3O4 nanoparticles for magnetically targeted drug delivery in ovarian cancer cells. J. Mater. Chem. B 2019, 7, 433–442. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, F.; Li, T.; Zhou, M.; Ye, J.; Ji, A.; Wang, H.; Ding, C.; Chen, H.; Xu, Z.; et al. Engineering Oxaliplatin Prodrug Nanoparticles for Second Near-Infrared Fluorescence Imaging-Guided Immunotherapy of Colorectal Cancer. Small 2021, 17, 2007882. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, L.; Guida, A.; Curigliano, G. Cetuximab for treating non-small cell lung cancer. Expert Opin. Biol. Ther. 2018, 18, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, T.; Morawski, P. Trastuzumab and its biosimilars. Pol. Merkur. Lekarski 2018, 44, 253–257. [Google Scholar]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Cengiz Seval, G.; Beksac, M. The safety of bortezomib for the treatment of multiple myeloma. Expert Opin. Drug Saf. 2018, 17, 953–962. [Google Scholar] [CrossRef]
- Heigener, D.F.; Reck, M. Crizotinib. Recent Results Cancer Res. 2018, 211, 57–65. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF -Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Lindauer, M.; Hochhaus, A. Dasatinib. Recent Results Cancer Res. 2018, 212, 29–68. [Google Scholar] [CrossRef]
- Suttorp, M.; Bornhäuser, M.; Metzler, M.; Millot, F.; Schleyer, E. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Expert Rev. Clin. Pharmacol. 2018, 11, 219–231. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. Recent Results Cancer Res. 2018, 211, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, B.N.; le Coutre, P.; Kim, T.D.; Quintás-Cardama, A. Nilotinib. Recent Results Cancer Res. 2014, 201, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 239–266. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.K. Vemurafenib. Recent Results Cancer Res. 2018, 211, 77–89. [Google Scholar] [CrossRef]
- Nejati, K.; Rastegar, M.; Fathi, F.; Dadashpour, M.; Arabzadeh, A.A. Nanoparticle-based drug delivery systems to overcome gastric cancer drug resistance. J. Drug Deliv. Sci. Technol. 2022, 70, 103231. [Google Scholar] [CrossRef]
- Fang, X.; Cao, J.; Shen, A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662. [Google Scholar] [CrossRef]
- Marcos, X.; Méndez-Luna, D.; Fragoso-Vázquez, M.J.; Rosales-Hernández, M.C.; Correa-Basurto, J. Anti-breast cancer activity of novel compounds loaded in polymeric mixed micelles: Characterization and in vitro studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102017. [Google Scholar] [CrossRef]
- Ioele, G.; De Luca, M.; Ragno, G. Photostability of barnidipine in combined cyclodextrin-in-liposome matrices. Future Med. Chem. 2014, 6, 35–43. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin. Drug Deliv. 2010, 7, 1399–1414. [Google Scholar] [CrossRef] [PubMed]
- Sauraj, V.; Kumar, B.; Deeba, F.; Bano, S.; Kulshreshtha, A.; Gopinath, P.; Negi, Y.S. Lipophilic 5-fluorouracil prodrug encapsulated xylan-stearic acid conjugates nanoparticles for colon cancer therapy. Int. J. Biol. Macromol. 2019, 128, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tucci, S.T.; Kheirolomoom, A.; Ingham, E.S.; Mahakian, L.M.; Tam, S.M.; Foiret, J.; Hubbard, N.E.; Borowsky, A.D.; Baikoghli, M.; Cheng, R.H.; et al. Tumor-specific delivery of gemcitabine with activatable liposomes. J. Control. Release 2019, 309, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Viudez, A.J.; Madueño, R.; Pineda, T.; Blázquez, M. Stabilization of Gold Nanoparticles by 6-Mercaptopurine Monolayers. Effects of the Solvent Properties. J. Phys. Chem. B 2006, 110, 17840–17847. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH guideline Q1A(R2). In ICH Harmonised Tripartite Guideline, Stability Testing of New Drug Substances and Products; European Medicines Agency: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Ragno, G.; Vetuschi, C.; Risoli, A.; Ioele, G. Application of a classical least-squares regression method to the assay of 1,4-dihydropyridine antihypertensives and their photoproducts. Talanta 2003, 59, 375–382. [Google Scholar] [CrossRef]
- Ragno, G.; Ioele, G.; De Luca, M.; Garofalo, A.; Grande, F.; Risoli, A. A critical study on the application of the zero-crossing derivative spectrophotometry to the photodegradation monitoring of lacidipine. J. Pharm. Biomed. Anal. 2006, 42, 39–45. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.; Monteiro, O.; Oliveira, M.C.; Florêncio, M.H. Photocatalytic degradation of cyclophosphamide and ifosfamide: Effects of wastewater matrix, transformation products and in silico toxicity prediction. Sci. Total Environ. 2019, 692, 503–510. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Karim, K.; Mandal, A.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, M.B.; Torrejais, J.; Janssens, R.; Luis, P.; Van der Bruggen, B.; Dubey, K.K.; Mandal, M.K.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J. Treatment of anticancer drugs in hospital and wastewater effluents using nanofiltration. Sep. Purif. Technol. 2019, 224, 273–280. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Padrón, M.E.T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Quantification of cytostatic platinum compounds in wastewater by inductively coupled plasma mass spectrometry after ion exchange extraction. Microchem. J. 2020, 157, 104862. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Hernández-Arencibia, P.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Simultaneous and systematic analysis of cytostatic drugs in wastewater samples by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1110–1111, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Toński, M.; Dołżonek, J.; Stepnowski, P.; Białk-Bielińska, A. Hydrolytic stability of anticancer drugs and one metabolite in the aquatic environment. Environ. Sci. Pollut. Res. Int. 2021, 28, 57939–57951. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Medina, A.; Sans, C.; Lacorte, S. Biological and photochemical degradation of cytostatic drugs under laboratory conditions. J. Hazard. Mater. 2017, 323, 319–328. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Campos, B.; Barata, C.; Lacorte, S. Degradation and toxicity of mitoxantrone and chlorambucil in water. Int. J. Environ. Sci. Technol. 2015, 12, 633–640. [Google Scholar] [CrossRef]
- Houot, M.; Poinsignon, V.; Mercier, L.; Valade, C.; Desmaris, R.; Lemare, F.; Paci, A. Physico-chemical stability of busulfan in injectable solutions in various administration packages. Drugs R D 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Goykhman, N.; Dror, I.; Berkowitz, B. Transport of platinum-based pharmaceuticals in water-saturated sand and natural soil: Carboplatin and cisplatin species. Chemosphere 2019, 219, 390–399. [Google Scholar] [CrossRef]
- Roque-Diaz, Y.; Sanadar, M.; Han, D.; López-Mesas, M.; Valiente, M.; Tolazzi, M.; Melchior, A.; Veclani, D. The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes 2021, 9, 1873. [Google Scholar] [CrossRef]
- Secrétan, P.H.; Karoui, M.; Sadou-Yaye, H.; Levi, Y.; Tortolano, L.; Solgadi, A.; Yagoubi, N.; Do, B. Imatinib: Major photocatalytic degradation pathways in aqueous media and the relative toxicity of its transformation products. Sci. Total Environ. 2019, 655, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Singh, P.; Mehrotra, R. Evaluation of Stability of 5- Fluorouracil under Different Stress Conditions: High Performance Liquid Chromatography and Infrared Spectroscopic Approach. Curr. Pharm. Anal. 2012, 8, 49–55. [Google Scholar] [CrossRef]
- Redasani, V.K.; Bari, S.B. Prodrug Design: Perspectives, Approaches and Applications in Medicinal Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128035573. [Google Scholar]
- Jornada, D.H.; Dos Santos Fernandes, G.F.; Chiba, D.E.; De Melo, T.R.F.; Dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2015, 21, 42. [Google Scholar] [CrossRef]
- Mucha, O.; Podkalicka, P.; Mikulski, M.; Barwacz, S.; Andrysiak, K.; Biela, A.; Mieczkowski, M.; Kachamakova-Trojanowska, N.; Ryszawy, D.; Białas, A.; et al. Development and characterization of a new inhibitor of heme oxygenase activity for cancer treatment. Arch. Biochem. Biophys. 2019, 671, 130–142. [Google Scholar] [CrossRef]
- Kumar, N.; Sangeetha, D.; Reddy, S.P. UPLC and LC–MS Studies on Degradation Behavior of Irinotecan Hydrochloride and Development of a Validated Stability-Indicating Ultra-Performance Liquid Chromatographic Method for Determination of Irinotecan Hydrochloride and its Impurities in Pharmaceutical Dosage Forms. J. Chromatogr. Sci. 2012, 50, 810–819. [Google Scholar] [CrossRef]
- Smith, J.A.; Morris, A.; Duafala, M.E.; Bertino, J.R.; Markman, M.; Kleinberg, M. Stability of floxuridine and leucovorin calcium admixtures for intraperitoneal administration. Am. J. Hosp. Pharm. 1989, 46, 985–989. [Google Scholar] [CrossRef]
- Walker, S.E.; Law, S.; Puodziunas, A. Simulation of Y-site compatibility of irinotecan and leucovorin at room temperature in 5% dextrose in water in 3 different containers. Can. J. Hosp. Pharm. 2005, 58, 212–222. [Google Scholar]
- Tashiro, M.; Naito, T.; Yamamoto, C.; Katoh, S.Y.; Kawakami, J. Impact of Light Shielding on Photo-Degradation of Dacarbazine during the Preparation Process. Biol. Pharm. Bull. 2019, 42, 2062–2068. [Google Scholar] [CrossRef]
- Le Basle, Y.; Chennell, P.; Tokhadze, N.; Astier, A.; Sautou, V. Physicochemical Stability of Monoclonal Antibodies: A Review. J. Pharm. Sci. 2020, 109, 169–190. [Google Scholar] [CrossRef]
- Shire, S.J. Stability of monoclonal antibodies (mAbs). Monoclon. Antibodies 2015, 6355, 45–92. [Google Scholar] [CrossRef]
- Paul, M.; Vieillard, V.; Jaccoulet, E.; Astier, A. Long-term stability of diluted solutions of the monoclonal antibody rituximab. Int. J. Pharm. 2012, 436, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S. Liquid formulation for antibody drugs. Biochim. Biophys. Acta 2014, 1844, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; O’Dell, C.; Bolessa, E.; McLinden, S.; Fortin, L.; Deorkar, N. Viscosity Reduction and Stability Enhancement of Monoclonal Antibody Formulations Using Derivatives of Amino Acids. J. Pharm. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bommana, R.; Chai, Q.; Schöneich, C.; Weiss, W.F.; Majumdar, R. Understanding the Increased Aggregation Propensity of a Light-Exposed IgG1 Monoclonal Antibody Using Hydrogen Exchange Mass Spectrometry, Biophysical Characterization, and Structural Analysis. J. Pharm. Sci. 2018, 107, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, J.; Salmerón-García, A.; Cabeza, J.; Vélez, C.; Capitán-Vallvey, L.F.; Navas, N. The Effects of Light-Accelerated Degradation on the Aggregation of Marketed Therapeutic Monoclonal Antibodies Evaluated by Size-Exclusion Chromatography With Diode Array Detection. J. Pharm. Sci. 2016, 105, 1405–1418. [Google Scholar] [CrossRef]
- Hernández-Jiménez, J.; Martínez-Ortega, A.; Salmerón-García, A.; Cabeza, J.; Prados, J.C.; Ortíz, R.; Navas, N. Study of aggregation in therapeutic monoclonal antibodies subjected to stress and long-term stability tests by analyzing size exclusion liquid chromatographic profiles. Int. J. Biol. Macromol. 2018, 118, 511–524. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.; Herrera, A.; Salmerón-García, A.; Cabeza, J.; Perez-Robles, R.; Navas, N. Degradation and in-use stability study of five marketed therapeutic monoclonal antibodies by generic weak cation exchange liquid chromatographic method ((WCX)HPLC/DAD). J. Chromatogr. B 2022, 1203, 123295. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortega, A.; Herrera, A.; Salmerón-García, A.; Cabeza, J.; Cuadros-Rodríguez, L.; Navas, N. Validated reverse phase HPLC diode array method for the quantification of intact bevacizumab, infliximab and trastuzumab for long-term stability study. Int. J. Biol. Macromol. 2018, 116, 993–1003. [Google Scholar] [CrossRef]
- Qiu, C.; Arzhantsev, S. Secondary structure assessment of formulated bevacizumab in the presence of SDS by deep ultraviolet resonance Raman (DUVRR) spectroscopy. Anal. Biochem. 2018, 555, 26–32. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed.Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Grande, F.; De Luca, M.; Occhiuzzi, M.A.; Garofalo, A.; Ragno, G. Photodegradation of Anti-Inflammatory Drugs: Stability Tests and Lipid Nanocarriers for Their Photoprotection. Molecules 2021, 26, 5989. [Google Scholar] [CrossRef] [PubMed]
- Salman, D.; Barton, S.; Gebara, S.N. Improving the stability of anticancer drugs. J. Oncol. Pharm. Pract. 2014, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.; Sarfraz, R.M.; Mahmood, A.; Hussain, Z.; Thu, H.E.; Zafar, N.; Ashraf, M.U.; Batool, N. Smart pH-responsive Co-polymeric Hydrogels for Controlled Delivery of Capecitabine: Fabrication, Optimization and In Vivo Toxicology Screening. Curr. Drug Deliv. 2021, 18, 1256–1271. [Google Scholar] [CrossRef]
- Rivero, C.W.; De Benedetti, E.C.; Sambeth, J.; Trelles, J.A. Biotransformation of cladribine by a nanostabilized extremophilic biocatalyst. J. Biotechnol. 2020, 323, 166–173. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Muthusamy, N.; Byrd, J.C.; Lee, R.J. Liposomal coencapsulated fludarabine and mitoxantrone for lymphoproliferative disorder treatment. J. Pharm. Sci. 2008, 97, 1508–1518. [Google Scholar] [CrossRef]
- Minhas, M.U.; Abdullah, O.; Sohail, M.; Khalid, I.; Ahmad, S.; Khan, K.U.; Badshah, S.F. Synthesis of novel combinatorial drug delivery system (nCDDS) for co-delivery of 5-fluorouracil and leucovorin calcium for colon targeting and controlled drug release. Drug Dev. Ind. Pharm. 2021, 47, 1952–1965. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Joladarashi, D.; Hallur, R.L.S.; Sanganal, J.S.; Phani, A.R. Toxicity evaluation of 6-mercaptopurine-Chitosan nanoparticles in rats. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 147–154. [Google Scholar] [CrossRef]
- Dorniani, D.; bin Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation and characterization of 6-mercaptopurine-coated magnetite nanoparticles as a drug delivery system. Drug Des. Devel. Ther. 2013, 7, 1015. [Google Scholar] [CrossRef]
- Dhanka, M.; Shetty, C.; Srivastava, R. Methotrexate loaded gellan gum microparticles for drug delivery. Int. J. Biol. Macromol. 2018, 110, 346–356. [Google Scholar] [CrossRef]
- Mishra, M.K.; Gupta, J.; Gupta, R. Self-Assemble Amphiphilic PEO-PPO-PEO Tri-Block Co-Polymeric Methotrexate Nanomicelles to Combat MCF7 Cancer Cells. Curr. Drug Deliv. 2021, 18, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Sierpe, R.; Noyong, M.; Simon, U.; Aguayo, D.; Huerta, J.; Kogan, M.J.; Yutronic, N. Construction of 6-thioguanine and 6-mercaptopurine carriers based on βcyclodextrins and gold nanoparticles. Carbohydr. Polym. 2017, 177, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, S.; Samadizadeh, M.; Khaleghian, M.; Zabarjad Shiraz, N. Theoretical study of encapsulation of Floxuridine anticancer drug into BN (9,9-7) nanotube for medical application. Phosphorus. Sulfur. Silicon Relat. Elem. 2019, 195, 293–306. [Google Scholar] [CrossRef]
- Xue, H.F.; Huang, Y.; Dong, M.; Zhang, Z.Y.; Li, C. Stabilization of Antitumor Agent Busulfan through Encapsulation within a Water-Soluble Pillar[5]arene. Chem. Asian J. 2022, 17, e202101332. [Google Scholar] [CrossRef]
- Khorram, R.; Raissi, H.; Morsali, A.; Shahabi, M. The computational study of the γ-Fe 2 O 3 nanoparticle as Carmustine drug delivery system: DFT approach. J. Biomol. Struct. Dyn. 2019, 37, 454–464. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, J.; Wang, K.; Tang, Y.; Zhang, X.; Zhang, H.; Huang, F.; Pei, Y.; Jiang, Y. Cationic core-shell nanoparticles with carmustine contained within O6-benzylguanine shell for glioma therapy. Biomaterials 2013, 34, 8968–8978. [Google Scholar] [CrossRef]
- Zhuang, L.; Gao, J.; Zeng, Y.; Yu, F.; Zhang, B.; Li, M.; Derendorf, H.; Liu, C. HPLC method validation for the quantification of lomustine to study pharmacokinetics of thermosensitive liposome-encapsulated lomustine containing iohexol for CT imaging in C6 glioma rats. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 61–69. [Google Scholar] [CrossRef]
- Ritschel, W.A.; Ye, W.; Buhse, L.; Reepmeyer, J.C. Stability of the nitrogen mustard mechlorethamine in novel formulations for dermatological use. Int. J. Pharm. 2008, 362, 67–73. [Google Scholar] [CrossRef]
- Tretiakova, D.; Le-Deigen, I.; Onishchenko, N.; Kuntsche, J.; Kudryashova, E.; Vodovozova, E. Phosphatidylinositol stabilizes fluid-phase liposomes loaded with a melphalan lipophilic prodrug. Pharmaceutics 2021, 13, 473. [Google Scholar] [CrossRef]
- Petre, C.E.; Dittmer, D.P.; Ellen, M.; Bldg, J. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 277. [Google Scholar]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Metwally, A.A.; Fahmy, R.H.; Osman, R. Chitosan-coated nanodiamonds: Mucoadhesive platform for intravesical delivery of doxorubicin. Carbohydr. Polym. 2020, 245, 116528. [Google Scholar] [CrossRef] [PubMed]
- Schilt, Y.; Berman, T.; Wei, X.; Nativ-Roth, E.; Barenholz, Y.; Raviv, U. Effect of the ammonium salt anion on the structure of doxorubicin complex and PEGylated liposomal doxorubicin nanodrugs. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129849. [Google Scholar] [CrossRef] [PubMed]
- Spindeldreier, K.C.; Thiesen, J.; Krämer, I. Loading, release and stability of epirubicin-loaded drug-eluting beads. J. Oncol. Pharm. Pract. 2016, 22, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, J.; Li, X.; Han, X.; Zou, Q.; Zhang, P.; Luo, Y.; Jin, Y. Preparation and pharmacokinetics of bifunctional epirubicin-loaded micelles. Pharmazie 2019, 74, 577–582. [Google Scholar] [CrossRef]
- Lu, E.; Shao, G.; Ma, J.; He, Y.; Gong, Y.; Yan, Z.; Sha, X. Optimized Loading of Idarubicin in CalliSpheres® Drug-Eluting Beads and Characterization of Release Profiles and Morphological Properties. Pharmaceutics 2021, 13, 799. [Google Scholar] [CrossRef]
- Guiu, B.; Hincapie, G.; Thompson, L.; Wu, Y.; Boulin, M.; Cassinotto, C.; Cruise, G.M. An In Vitro Evaluation of Four Types of Drug-Eluting Embolics Loaded with Idarubicin. J. Vasc. Interv. Radiol. 2019, 30, 1303–1309. [Google Scholar] [CrossRef]
- Xu, G.; Tang, H.; Chen, J.; Zhu, M.; Xie, Y.; Li, Y.; Hao, Q.; Sun, Y.; Cong, D.; Meng, Q.; et al. Estrone-targeted liposomes for mitoxantrone delivery via estrogen receptor: In vivo targeting efficacy, antitumor activity, acute toxicity and pharmacokinetics. Eur. J. Pharm. Sci. 2021, 161, 1303–1309. [Google Scholar] [CrossRef]
- Sargazi, A.; Shiri, F.; Keikha, S.; Majd, M.H. Hyaluronan magnetic nanoparticle for mitoxantrone delivery toward CD44-positive cancer cells. Colloids Surf. B. Biointerfaces 2018, 171, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Qi, Q.; Mao, Z.; Zhan, X. PLGA nanoparticles introduction into mitoxantrone-loaded ultrasound-responsive liposomes: In vitro and in vivo investigations. Int. J. Pharm. 2017, 528, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorg. Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Tahover, E.; Kornev, G.; Patil, Y.; Amitay, Y.; Ohana, P.; Sapir, E.; Zalipsky, S. Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside. Adv. Drug Deliv. Rev. 2020, 154–155, 13–26. [Google Scholar] [CrossRef]
- Yadav, K.; Sawant, K. Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation. Curr. Drug Deliv. 2010, 7, 51–64. [Google Scholar] [CrossRef]
- Jiang, H.; Pei, L.; Liu, N.; Li, J.; Li, Z.; Zhang, S. Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy. Drug Deliv. 2016, 23, 1379–1382. [Google Scholar] [CrossRef]
- Son, K.; Alkan-Onyuksel, H. Stabilization of Teniposide in Aqueous Mixtures of Detergent-Phospholipid. PDA J. Pharm. Sci. Technol. 1996, 50, 366–371. [Google Scholar]
- He, S.; Yang, H.; Zhang, R.; Li, Y.; Duan, L. Preparation and in vitro-in vivo evaluation of teniposide nanosuspensions. Int. J. Pharm. 2015, 478, 131–137. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Q.; Gan, T.; Fang, Y.; Yue, P.; Sun, Y.; Jin, Y.; Feng, J.; Tu, L. Nanocrystal-Loaded Micelles for the Enhanced In Vivo Circulation of Docetaxel. Molecules 2021, 26, 4481. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, N.-W.; Kim, H.; Kim, D.H.; Chae, J.-W.; Lee, W.; Song, G.Y.; Cho, C.-W.; Kim, D.-D.; Lee, J.-Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253. [Google Scholar] [CrossRef]
- Sun, B.; Jing, H.; Mabrouk, M.T.; Zhang, Y.; Jin, H.; Lovell, J.F. A surfactant-stripped cabazitaxel micelle formulation optimized with accelerated storage stability. Pharm. Dev. Technol. 2020, 25, 4481. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, R.J.; Meng, F.; Wang, G.; Zheng, X.; Dong, S.; Teng, L. Microfluidic self-assembly of high cabazitaxel loading albumin nanoparticles. Nanoscale 2020, 12, 16928–16933. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Liang, M.X.; Wu, Y.; Yang, K.; Tang, J.H.; Zhang, W. Extracellular vesicles as drug vectors for precise cancer treatment. Nanomedicine 2021, 16, 1519–1537. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Zhou, J.; Xie, Z. Merocyanine-paclitaxel conjugates for photothermal induced chemotherapy. J. Mater. Chem. B 2021, 9, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Hao, D.; Li, C.; Lu, S.; Pei, Q.; Xie, Z. Fluorinated paclitaxel prodrugs for potentiated stability and chemotherapy. J. Mater. Chem. B 2021, 9, 9971–9979. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Ahmadvand, H.; Farhadi, A.; Najmafshar, A.; Chiani, M.; Norouzian, D. Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells. Drug Dev. Ind. Pharm. 2018, 44, 1371–1376. [Google Scholar] [CrossRef]
- Li, M.; Ma, S.; Xie, X.; Liu, N.; Li, Z.; Yang, Z.; Gao, G.; Li, S.; Li, Y.; Li, S.; et al. Vincristine-doxorubicin co-loaded artificial low-density lipoproteins towards solid tumours. Eur. J. Med. Chem. 2021, 226, 113802. [Google Scholar] [CrossRef]
- Mao, W.; Wu, F.; Lee, R.J.; Lu, W.; Wang, J. Development of a stable single-vial liposomal formulation for vincristine. Int. J. Nanomed. 2019, 14, 4461–4474. [Google Scholar] [CrossRef]
- Li, C.; Cui, J.; Wang, C.; Cao, J.; Zhang, L.; Li, Y.; Liang, M.; Xiu, X.; Li, Y.; Wei, N.; et al. Sulfosalicylate mediates improved vinorelbine loading into LUVs and antineoplastic effects. J. Liposome Res. 2012, 22, 42–54. [Google Scholar] [CrossRef]
- Bahadori, F.; Topçu, G.; Eroğlu, M.S.; Önyüksel, H. A new lipid-based nano formulation of vinorelbine. AAPS PharmSciTech 2014, 15, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gou, J.; Wang, Y.; Tan, X.; Zhao, L.; Jin, X.; Tang, X. Synergistic Antitumor Efficacy Mediated by Liposomal Co-Delivery of Polymeric Micelles of Vinorelbine and Cisplatin in Non-Small Cell Lung Cancer. Int. J. Nanomed. 2021, 16, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, W.; Yan, H.; Liu, H.; Wang, C. Development of intravenous lipid emulsion of vinorelbine based on drug-phospholipid complex technique. Int. J. Pharm. 2013, 454, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, L.; Li, Y.; Wang, H.; Xu, S.; Zhang, X.; Wu, R.; Yang, G. Superparamagnetic chitosan nanocomplexes for colorectal tumor-targeted delivery of irinotecan. Int. J. Pharm. 2020, 584, 119394. [Google Scholar] [CrossRef]
- Delrish, E.; Jabbarvand, M.; Ghassemi, F.; Amoli, F.A.; Atyabi, F.; Lashay, A.; Soleimani, M.; Aghajanpour, L.; Dinarvand, R. Efficacy of topotecan nanoparticles for intravitreal chemotherapy of retinoblastoma. Exp. Eye Res. 2021, 204, 108423. [Google Scholar] [CrossRef]
- Souza, L.G.; Silva, E.J.; Martins, A.L.L.; Mota, M.F.; Braga, R.C.; Lima, E.M.; Valadares, M.C.; Taveira, S.F.; Marreto, R.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, K.; Li, C.; Zhang, X.; Wu, W.; Jiang, X. Cisplatin-Rich Polyoxazoline-Poly(aspartic acid) Supramolecular Nanoparticles. Macromol. Biosci. 2017, 17, 1700206. [Google Scholar] [CrossRef]
- Alavi, S.E.; Raza, A.; Koohi Moftakhari Esfahani, M.; Akbarzadeh, A.; Abdollahi, S.H.; Ebrahimi Shahmabadi, H. Carboplatin Niosomal Nanoplatform for Potentiated Chemotherapy. J. Pharm. Sci. 2022. [Google Scholar] [CrossRef]
- Masuda, R.; Hayashi, R.; Nose, H.; Taguchi, A.; Hayashi, Y.; Yasui, H.; Koide, T. Development of a carboplatin derivative conjugated with a collagen-like triple-helical peptide. Future Med. Chem. 2018, 10, 619–629. [Google Scholar] [CrossRef]
- Liang, S.; Han, L.; Mu, W.; Jiang, D.; Hou, T.; Yin, X.; Pang, X.; Yang, R.; Liu, Y.; Zhang, N. Carboplatin-loaded SMNDs to reduce GSH-mediated platinum resistance for prostate cancer therapy. J. Mater. Chem. B 2018, 6, 7004–7014. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Liu, M.; Jin, D.; Zhang, L.; Cheng, J.; Liu, Y. Conjugation of oxaliplatin with PEGylated-nanobody for enhancing tumor targeting and prolonging circulation. J. Inorg. Biochem. 2021, 223, 111553. [Google Scholar] [CrossRef] [PubMed]
- Giannos, S.A.; Kraft, E.R.; Zhao, Z.Y.; Merkley, K.H.; Cai, J. Formulation Stabilization and Disaggregation of Bevacizumab, Ranibizumab and Aflibercept in Dilute Solutions. Pharm. Res. 2018, 35, 78. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Sapino, S.; Chindamo, G.; Oliaro-bosso, S.; Adinolfi, S.; Dianzani, C.; Baratta, F.; Gallarate, M. A New Bevacizumab Carrier for Intravitreal Administration: Focus on Stability. Pharmaceutics 2021, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Cruz, A.; Pinto, I.M.; Sarmento, B. Nanoparticles provide long-term stability of bevacizumab preserving its antiangiogenic activity. Acta Biomater. 2018, 78, 285–295. [Google Scholar] [CrossRef]
- Alves, A.; Bruinsmann, F.; Guterres, S.; Pohlmann, A. Organic Nanocarriers for Bevacizumab Delivery: An Overview of Development, Characterization and Applications. Molecules 2021, 26, 4127. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wei, M.; Liu, C.; Yang, J. Cetuximab-modified silica nanoparticle loaded with ICG for tumor-targeted combinational therapy of breast cancer. Drug Deliv. 2019, 26, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Viswanadh, M.K.; Vikas; Jha, A.; Reddy Adena, S.K.; Mehata, A.K.; Priya, V.; Neogi, K.; Poddar, S.; Mahto, S.K.; Muthu, M.S. Formulation and in vivo efficacy study of cetuximab decorated targeted bioadhesive nanomedicine for non-small-cell lung cancer therapy. Nanomedicine 2020, 15, 2345–2367. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, Y.; Wei, Y.; Haag, R.; Sun, H.; Zhong, Z. Cetuximab-Polymersome-Mertansine Nanodrug for Potent and Targeted Therapy of EGFR-Positive Cancers. Biomacromolecules 2022, 23, 100–111. [Google Scholar] [CrossRef]
- Song, L.; Chen, Y.; Ding, J.; Wu, H.; Zhang, W.; Ma, M.; Zang, F.; Wang, Z.; Gu, N.; Zhang, Y. Rituximab conjugated iron oxide nanoparticles for targeted imaging and enhanced treatment against CD20-positive lymphoma. J. Mater. Chem. B 2020, 8, 895–907. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-Coated Nanoparticles Loaded With Docetaxel for Breast Cancer Therapy. Dose Response 2019, 17, 1559325819872583. [Google Scholar] [CrossRef]
- Rodallec, A.; Brunel, J.M.; Giacometti, S.; Maccario, H.; Correard, F.; Mas, E.; Orneto, C.; Savina, A.; Bouquet, F.; Lacarelle, B.; et al. Docetaxel-trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451–3465. [Google Scholar] [CrossRef] [PubMed]
- Reslan, M.; Ranganathan, V.; Macfarlane, D.R.; Kayser, V. Choline ionic liquid enhances the stability of Herceptin® (trastuzumab). Chem. Commun. 2018, 54, 10622–10625. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Jang, S.J.; Seo, M.J.; Park, J.H.; Kim, B.S.; Kim, E.J.; Lee, Y.J.; Lee, T.S.; An, G.I.; Song, I.H.; et al. Development of 64 Cu-NOTA-Trastuzumab for HER2 Targeting: A Radiopharmaceutical with Improved Pharmacokinetics for Human Studies. J. Nucl. Med. 2019, 60, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.J.; Feng, B.; Shen, J.; Zhang, M.; Hu, Y.Q.; Jiang, A.X.; Zhu, D.Q.; Chen, Y.W.; Ji, W.; Zhang, Z.; et al. An Avascular Niche Created by Axitinib-Loaded PCL/Collagen Nanofibrous Membrane Stabilized Subcutaneous Chondrogenesis of Mesenchymal Stromal Cells. Adv. Sci. 2021, 8, 2100351. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Sahoo, R.K.; Nakhate, K.T.; Ajazuddin; Gupta, U. Biotinylated HPMA centered polymeric nanoparticles for Bortezomib delivery. Int. J. Pharm. 2020, 579, 2100351. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Du, C.; Li, M.; Li, S.; Galons, H.; Guo, N.; Yu, P. Co-delivery of F7 and crizotinib by thermosensitive liposome for breast cancer treatment. J. Liposome Res. 2021. [Google Scholar] [CrossRef]
- Niza, E.; Noblejas-lópez, M.D.M.; Bravo, I.; Nieto-jiménez, C.; Castro-osma, J.A.; Canales-vázquez, J.; Lara-sanchez, A.; Moya, E.M.G.; Burgos, M.; Ocaña, A.; et al. Trastuzumab-Targeted Biodegradable Nanoparticles for Enhanced Delivery of Dasatinib in HER2+ Metastasic Breast Cancer. Nanomaterials 2019, 9, 1793. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Sultan, M.H.; Albarraq, A.A.; Ahmed, R.A.; Alhazmi, H.A.; Alam, M.I. Preparation, Characterization, and Anticancer Activity of Nanostructured Lipid Carriers Containing Imatinib. Pharmaceutics 2021, 13, 1086. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Wang, S.; Ouyang, L.; Li, H.; Ding, J.; Deng, G.; Zhou, W. Imatinib co-loaded targeted realgar nanocrystal for synergistic therapy of chronic myeloid leukemia. J. Control. Release 2021, 338, 190–200. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.M.; Wang, D.L.; Du, J.L.; Guo, H.Y.; Chen, H.N.; Zhao, S.J.; Liu, Z.P.; Liu, Y. Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals. Molecules 2020, 25, 604. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, F.; Du, C.; Wang, H.; Mahato, R.I.; Huang, Y. Doxorubicin and lapatinib combination nanomedicine for treating resistant breast cancer. Mol. Pharm. 2014, 11, 2600–2611. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zheng, X.; Pang, X.; Pang, Z.; Zhao, J.; Zhang, Z.; Jiang, T.; Xu, W.; Zhang, Q.; Jiang, X. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 2016, 7, 34038–34051. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, X.; Pang, X.; Zhang, Z.; Zhang, Q. Incorporation of lapatinib into human serum albumin nanoparticles with enhanced anti-tumor effects in HER2-positive breast cancer. Colloids Surf. B Biointerfaces 2015, 136, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, S.; Chen, C.; Cao, S.; Yang, Z.; Pang, Z.; Xi, Z.; Pan, S.; Zhang, Q.; Jiang, X. Incorporation of lapatinib into lipoprotein-like nanoparticles with enhanced water solubility and anti-tumor effect in breast cancer. Nanomedicine 2013, 8, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Pepe, V.; Zappulla, C.; Cimino, C.; Pricoco, A.; Puglisi, G.; Giuliano, F.; Pignatello, R.; Carbone, C. Sorafenib repurposing for ophthalmic delivery by lipid nanoparticles: A preliminary study. Pharmaceutics 2021, 13, 1956. [Google Scholar] [CrossRef] [PubMed]
- Benizri, S.; Ferey, L.; Alies, B.; Mebarek, N.; Vacher, G.; Appavoo, A.; Staedel, C.; Gaudin, K.; Barthélémy, P. Nucleoside-Lipid-Based Nanocarriers for Sorafenib Delivery. Nanoscale Res. Lett. 2018, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Vanani, R.; Azarpira, N.; Heli, H.; Karimian, K.; Sattarahmady, N. A novel self-nanoemulsifying formulation for sunitinib: Evaluation of anticancer efficacy. Colloids Surf. B Biointerfaces 2017, 160, 65–72. [Google Scholar] [CrossRef]
- Qin, T.; Xu, X.; Zhang, Z.; Li, J.; You, X.; Guo, H.; Sun, H.; Liu, M.; Dai, Z.; Zhu, H. Paclitaxel/sunitinib-loaded micelles promote an antitumor response in vitro through synergistic immunogenic cell death for triple-negative breast cancer. Nanotechnology 2020, 31, 365101. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Alshetaili, A.S.; Alalaiwe, A.; Alsulays, B.B.; Anwer, M.K.; Al-Shdefat, R.; Imam, F.; Shakeel, F. Anticancer Efficacy of Self-Nanoemulsifying Drug Delivery System of Sunitinib Malate. AAPS PharmSciTech 2018, 19, 123–133. [Google Scholar] [CrossRef]
- Jáklová, K.; Feglarová, T.; Rex, S.; Heger, Z.; Eckschlager, T.; Hraběta, J.; Hodek, P.; Kolárik, M.; Indra, R. Apoferritin/Vandetanib Association Is Long-Term Stable But Does Not Improve Pharmacological Properties of Vandetanib. Int. J. Mol. Sci. 2021, 22, 4250. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]

| Drug Classes | Active Compound | Prodrug | Diseases | Ref. |
|---|---|---|---|---|
| Antimetabolites | Mercaptopurine | Azathioprine | Acute lymphoblastic leukemia | [6,7] |
| 5-Fluorouracil | Capecitabine | Breast cancer, esophageal cancer, laryngeal cancer, gastrointestinal and genitourinary tract cancer | [8] | |
| Deoxyadenosine | Cladribine | Hairy cell leukemia | [9,10] | |
| 1-β-D-arabinofuranoside 5′-triphosphate | Cytarabine | Acute myeloid leukemia | [11] | |
| 9-beta-D-arabinosyl-2-fluoroadenine | Fludarabine | Chronic lymphocytic leukemia | [11] | |
| 5-Fluorouracil | Different types of neoplasms | [8] | ||
| Gemcitabine diphosphate and triphosphate | Gemcitabine | Solid cancers | [11] | |
| 6-Mercaptopurine | Acute lymphoblastic leukemia | [7,11,12] | ||
| Methotrexate | Several kinds of cancer, such as colon cancer | [13] | ||
| 6-Thioguanosine | 6-Thioguanine | leukemias, lymphomas, mesothelioma, melanoma, biliary tract cancer, glioblastoma, osteosarcoma, soft tissue sarcoma, neuroendocrine tumors and lung, pancreatic and squamous cell carcinomas | [14,15] | |
| 5-Fluorouracil | Floxuridine | Liver cancer | [6,16] | |
| Methyl-tetrahydrofolate | Leucovorin | Acute lymphoblastic leukemia | [17,18] | |
| Alkylating agents | Busulfan | Chronic myelogenous leukemia | [19] | |
| Carmustine | Glioblastoma multiforme | [20] | ||
| Acrolein and phosphoramide mustard | Cyclophosphamide | Several kinds of cancer and autoimmune disorders | [21,22] | |
| 5-aminoimidazole-4-carboxamide | Dacarbazine | Malignant melanoma or sarcoma | [23] | |
| Lomustine | Brain tumors | [24] | ||
| Mechlorethamine | Mycosis fungoides | [25] | ||
| Melphalan | Multiple myeloma | [26] | ||
| Azo-Procarbazine | Procarbazine | Hodgkin’s lymphoma | [27,28] | |
| Triethylenethio-phosphoramide | Thiotepa | Ovarian cancer, breast cancer and superficial bladder cancer | [29,30] | |
| Semustine | Lewis lung carcinoma, leukemia, metastatic brain tumor, Hodgkin’s lymphoma, malignant melanoma and lung carcinoma | [31] | ||
| Anthracyclines | Daunorubicin | Leukemia | [32] | |
| Doxorubicin | Leukemia, breast cancer | [32] | ||
| Epirubicin | Breast cancer | [33] | ||
| Idarubicin | Acute leukemia | [34] | ||
| Mitoxantrone | Breast and prostate cancers, lymphomas and leukemias | [35] | ||
| Antitumor antibiotic | Bleomycin | Hodgkin’s and non-Hodgkin’s lymphoma, renal, cervical, laryngeal, testicular, lung and others | [36] | |
| Dactinomicyn | Different solid cancer | [37] | ||
| Mitomycin | Adenocarcinoma of the stomach | [38] | ||
| Plicamycin | Testicular and germ cancers | [39] | ||
| Epipodophyllotoxins | Etoposide | Small-cell lung cancer, leukemia, lymphoma, breast and ovarian carcinomas, testicular cancer | [40] | |
| Teniposide | Small-cell lung cancer, leukemia | [41] | ||
| Taxanes | Cabazitaxel | Prostatic cancer | [42] | |
| Docetaxel | Metastatic prostate cancer | [43] | ||
| Paclitaxel | Ovarian, breast and lung cancer, as well as Kaposi’s sarcoma | [44] | ||
| Vinca alkaloids | Vinblastine | Vinblastine-N-Oxide | Pancreatic ductal adenocarcinoma | [45] |
| Vincristine | Precursor B-cell acute lymphoblastic leukemia | [46] | ||
| Vinorelbine | Non-small-cell lung cancer and metastatic breast cancer | [47] | ||
| Campotothecins | SN-38 (7-ethyl-10-hydroxy-camptothecin) | Irinotecan | Solid tumors, including colorectal, pancreatic and lung cancer | [48] |
| Topotecan | Cervical cancer | [49] | ||
| Platinum analogs | Carboplatin | Ovarian cancer cells | [50] | |
| Cisplatin | Solid cancers, such as testicular, ovarian, head and neck, bladder, lung, cervical cancer, melanoma, lymphomas and several others | [50,51] | ||
| Oxaliplatin | Colorectal cancer | [52] | ||
| Monoclonal antibody | Bevacizumab | Metastatic colorectal cancer, metastatic breast cancer, non-small-cell lung cancer, glioblastoma, renal-cell carcinoma, ovarian cancer and cervical cancer | [53] | |
| Cetuximab | Non-small-cell lung cancer | [54] | ||
| Rituximab | Lymphoid malignancies, including aggressive forms of B-cell non-Hodgkin lymphoma, B-cell malignancies, follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia and mantle cell lymphoma | [55] | ||
| Trastuzumab | Breast and metastatic gastric cancer | [56] | ||
| Growth inhibitor | Axitinib | Renal-cell carcinoma | [57] | |
| Bortezomib | Multiple myeloma | [58] | ||
| Bosutinib | Philadelphia chromosome-positive chronic myelogenous leukemia | [6] | ||
| Crizotinib | Non-small-cell lung cancer | [59] | ||
| Dabrafenib | BRAF-mutated melanoma | [60] | ||
| Dasatinib | Chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia | [61] | ||
| Imatinib | Chronic myeloid leukemia (CML) | [62] | ||
| Lapatinib | Breast and gastrointestinal cancer | [63] | ||
| Nilotinib | Chronic myeloid leukemia (CML) | [64] | ||
| Pazopanib | Metastatic renal-cell carcinoma | [65] | ||
| Sorafenib | Hepatocellular carcinoma | [66] | ||
| Sunitinib | Renal-cell carcinoma | [57] | ||
| Trametinib | BRAF-mutated melanoma | [60] | ||
| Vandetanib | Metastatic medullary tyroid cancer | [67] | ||
| Vemurafenib | BRAF-mutated melanoma | [68] |
| Drug | Inclusion Systems | Advantages | Ref. |
|---|---|---|---|
| Capecitabine | Smart pH-responsive co-polymeric hydrogels | Protection from chemical and enzymatic hydrolysis and improvement in the stability in the gastric media | [118] |
| Cladribine | Nanostabilized polyacrylamide matrix | Better operational stability and mechanical properties | [119] |
| Cytarabine | Liposomal formulation in hydrogel system | Improvement in stability | [74] |
| Fludarabina | Co-encapsulation with mitoxantrone in liposomes | Improvement in long-term stability | [120] |
| 5-Fluorouracil | Co-encapsulation with leucovorin in NPs | Improvement in long-term stability | [75,121] |
| Gemcitabine | Temperature-sensitive liposomes | Improvement in long-term stability | [76] |
| 6-Mercaptopurine | NPs | Improvement in thermal stability | [122] |
| Gold NPs | Improvement in stability in diluted aqueous solutions | [77] | |
| Magnetite NPs | Improvement in thermal stability | [123] | |
| Methotrexate | Gellan gum microparticles | Higher thermal stability | [124] |
| Amphiphilic PEO–PPO–PEO tri-block co-polymeric nanomicelles | Improvement in thermodynamic stability | [125] | |
| 6-Thioguanine | Inclusion in βcyclodextrin and subsequent interaction with gold NPs | Increase in solubility and improvement in stability | [126] |
| Floxuridine | Boron nitride nanotube encapsulation | Improvement in long-term stability | [127] |
| Leucovorin | Co-encapsulation in NPs with of 5-fluorouracil | Improvement in long-term stability | [121] |
| Busulfan | Encapsulation within water-soluble pillae[5]arene | Reduction in hydrolytic degradation | [128] |
| Carmustine | Adsorption on the surface of the γ-Fe2O3 NPs | Improvement in long-term stability | [129] |
| Cationic core-shell NPs | Improvement in long-term stability | [130] | |
| Lomustine | Thermosensitive liposomes | Improvement in long-term stability | [131] |
| Mechlorethamine | Addition of free radical inhibitor for topical use | Improvement in long-term stability | [132] |
| Melphalan | Liposomal formulation based on a fluid lipid bilayer of natural phospholipids in the form of dioleoylglyceride ester | Improvement in stability in human serum | [133] |
| Daunorubicin | Liposomes | Improvement in long-term stability | [134,135] |
| Doxorubicin | Poly(lactide-co-glycolide) NPs with poloxamer 188 | Improvement in long-term stability | [136] |
| Peptide-based hydrogels and nanogels | Improvement in long-term stability | [137] | |
| Chitosan-coated nanodiamonds | Improvement in long-term stability | [138] | |
| PEGylated liposomal nanodrugs | Improvement in long-term stability | [139] | |
| Epirubicin | Drug-eluting beads | Improvement in long-term stability | [140] |
| Bifunctional drug-loaded micelles | Improvement in long-term stability | [141] | |
| Idarubicin | Drug-eluting beads | Improvement in long-term stability | [142] |
| Drug-eluting embolics beads | Improvement in long-term stability | [143] | |
| Mitoxantrone | Estrone-targeted liposomes | Improvement in long-term stability | [144] |
| Hyaluronan magnetic NPs | Improvement in long-term stability | [145] | |
| Liposomes in PLGA NPs | Improvement in long-term stability | [146] | |
| Bleomycin | Biodegradable chitosan nanogel | Improvement in thermal stability | [147] |
| Mitomycin | PEGylated liposomes | Improvement in long-term stability | [148] |
| Etoposide | PLGA NPs | Improvement in long-term stability | [149] |
| Nanostructured lipid carriers | Improvement in long-term stability | [150] | |
| Teniposide | Aqueous mixtures of detergent-phospholipid | Improvement in long-term stability | [151] |
| Nanosuspensions | Improvement in long-term stability | [152] | |
| Docetaxel | Nanocrystal-loaded micelles | Enhancement in blood circulation | [153] |
| Chondroitin sulphate-hybridized zein NPs | Improvement in long-term stability | [154] | |
| Cabazitaxel | Surfactant-stripped micelles | Improvement in long-term stability | [155] |
| Albumin NPs | Improvement in long-term stability | [156] | |
| Paclitaxel | Natural exosome | Improvement in stability profile | [157] |
| Polymeric micellar system | Increased solubility, greater stability | [158] | |
| Merocyanine conjugates | Favorable biological stability | [159] | |
| 17-fluorinated ethanol-modified drug in NPs | Robust colloidal stability | [160] | |
| Vinblastine | PEGylated niosomes | Increased solubility in water, reduction in side effects | [161] |
| Vincristine | Artificial low-density lipoproteins | Improvement in diffusion capacity in tumor tissue and lower toxicity | [162] |
| Liposomes | Improvement in efficacy stability | [163] | |
| Vinorelbine | Liposomes prepared with ammonium salts of several anionic agents | Improvement in efficacy and stability | [164] |
| Nanomicelles | Reduction in side effects and increase in drug efficacy | [165] | |
| Liposome encapsulating polymeric micelles. Co-encapsulation with cis-diamminedichloroplatinum (II) | Reduction in toxicity and increase in plasma half-life | [166] | |
| Intravenous lipid emulsion | Improvement in lipophilicity, and fewer toxic effects | [167] | |
| Irinotecan | Superparamagnetic chitosan nanocomplex | Improvement in effectiveness and biodistribution | [168] |
| Topotecan | Thiolated chitosan NPs | Improvement in stability and increase in absorption | [169] |
| Lipid NPs | Protection from hydrolysis | [170] | |
| Cisplatin | Liposome encapsulating polymeric micelles. Co-encapsulation with vinorelbine | Reduction in toxicity and increase in plasma half-life | [166] |
| NPs | Improvement in stability | [171] | |
| Carboplatin | Niosomal nanoplatform | Improvement in stability | [172] |
| Conjugation with an arginine-rich triple-helical peptide | Improvement in pharmacokinetic profile | [173] | |
| NPs | Outstanding plasma stability | [174] | |
| Oxaliplatin | Conjugation with PEGylated-nanobody | Prolonged circulation in vivo | [175] |
| Bevacizumab | Excipient in dilute solutions | Stabilization in unfavorable conditions, such as low concentration or body temperature. Prevention of aggregation. | [176,177] |
| Lipid NPs | Biochemical and biophysical stabilization. Prevention of aggregation. | [178] | |
| Nanoincapsulation into PLGA NPs | Improvement in long-term stability. Prevention of aggregation. | [179] | |
| Cetuximab | Silica NPs | Improvement in stability and bioavailability. Prevention of aggregation. | [180] |
| Chitosan NPs with and without drug conjugation | Improvement in stability and bioavailability. Prevention of aggregation. | [181] | |
| Polymersome–mertansine nanodrug | Improvement in stability and bioavailability. Prevention of aggregation. | [182] | |
| Rituximab | Iron oxide NPs | Colloidal stability in buffer solution. Prevention of aggregation. | [183] |
| Trastuzumab | Coated NPs with docetaxel | Prevention of aggregation and improvement in stability and pharmacokinetics profile | [184] |
| Stealth immunoliposome coated with docetaxel | Prevention of aggregation and improvement in stability and pharmacokinetics profile | [185] | |
| Choline ionic liquid vesicles | Prevention of aggregation and improvement in stability and pharmacokinetics profile | [186] | |
| Drug conjugated with SCN-Bn-NOTA and radiolabeled with 64Cu | Prevention of aggregation and improvement in stability and pharmacokinetics profile | [187] | |
| Axitinib | Nanofibrous membranes prepared with poly(ε-caprolactone)/collagen | Improvement in long-term stability | [188] |
| Bortezomib | Polymeric NPs | Improvement in water solubility chemical stability | [189] |
| Crizotinib | Thermosensitive liposome | Improvement in targeting efficacy | [190] |
| Dasatinib | Biodegradable NPs | Improvement in long-term stability | [191] |
| H-sensitive targeted micelle system. Co-encapsulation with curcumin | Improvement in long-term stability | [192] | |
| Imatinib | Nanostructured lipid carriers | Improvement in long-term stability at 25 °C | [193] |
| Nanocrystal delivery system | Improvement in long-term stability | [194] | |
| Lapatinib | Nanocrystals stabilized with a PEG coating | Improvement in stability for at least 4 days in plasma-containing buffers | [195] |
| Polymeric micelles | Improvement in stability | [196] | |
| Human serum albumin NPs | Improvement in stability | [197,198] | |
| Incorporation in lipoprotein-like NPs | Improvement in solubility in water and organic solvents | [199] | |
| Sorafenib | Solid lipid NPs | Increase in homogeneity and improvement in physical stability | [200] |
| Nucleoside-lipid-based nanocarriers | Increase in homogeneity and improvement in physical stability | [201] | |
| Sunitinib | Self-nanoemulsifying system | Improvement in long-term stability | [202] |
| Paclitaxel-loaded micelles | Improvement in long-term stability | [203] | |
| Self-nanoemulsifying system | Improvement in long-term stability | [204] | |
| Vandetanib | Nanocarrier based on apoferritin | Improvement in drug delivery | [205] |
| Vemurafenib | Peptide-modified loaded liposomes | Improvement in long-term stability | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. https://doi.org/10.3390/molecules27175436
Ioele G, Chieffallo M, Occhiuzzi MA, De Luca M, Garofalo A, Ragno G, Grande F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules. 2022; 27(17):5436. https://doi.org/10.3390/molecules27175436
Chicago/Turabian StyleIoele, Giuseppina, Martina Chieffallo, Maria Antonietta Occhiuzzi, Michele De Luca, Antonio Garofalo, Gaetano Ragno, and Fedora Grande. 2022. "Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties" Molecules 27, no. 17: 5436. https://doi.org/10.3390/molecules27175436
APA StyleIoele, G., Chieffallo, M., Occhiuzzi, M. A., De Luca, M., Garofalo, A., Ragno, G., & Grande, F. (2022). Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules, 27(17), 5436. https://doi.org/10.3390/molecules27175436