Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reactions between 1,4-Naphthoquinones and N-Acetyl-L-Cysteine
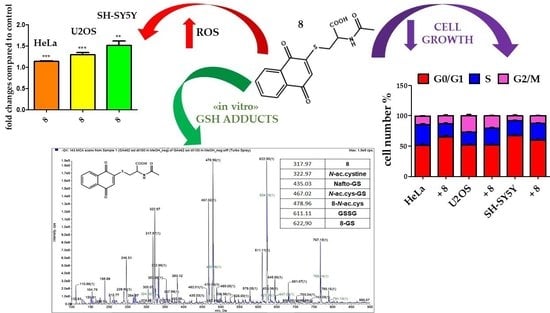
2.2. Biological Activity
2.2.1. Redox Imbalance
2.2.2. Effect on Cell Proliferation
2.2.3. Product Reactivity with Glutathione
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. General
3.1.2. General Procedure
3.2. Biology
3.2.1. Cell Culture and Treatments
3.2.2. MTT Assay
3.2.3. Measurement of Intracellular ROS Level
3.2.4. Cell-Cycle Analysis via Flow Cytometry
3.2.5. Reactions with GSH and DI-MS Analysis
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Souza, A.S.; Ribeiro, R.C.B.; Costa, D.C.S.; Pauli, F.P.; Pinho, D.R.; De Moraes, M.G.; Da Silva, F.C.; Forei, L.D.S.M.; Ferreira, V.F. Menadione: A platform and a target to valuable compounds synthesis. Beilst. J. Org. Chem. 2022, 18, 381–419. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Rappoport, Z. (Eds.) The Quinonoid Compounds: Part 1; John Wiley & Sons Ltd.: New York, NY, USA, 1988; Volume 1. [Google Scholar] [CrossRef]
- Patai, S.; Rappoport, Z. (Eds.) The Quinonoid Compounds: Part 2; John Wiley & Sons Ltd.: New York, NY, USA, 1988; Volume 2. [Google Scholar] [CrossRef]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Waste Biomass-Valorization 2021, 12, 6339–6374. [Google Scholar] [CrossRef]
- Boga, C.; Delpivo, C.; Ballarin, B.; Morigi, M.; Galli, S.; Micheletti, G.; Tozzi, S. Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing. Dyes Pigments 2013, 97, 9–18. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, M.; Ludynia, M.; Karcz, W. The Effect of Naphthazarin on the Growth, Electrogenicity, Oxidative Stress, and Microtubule Array in Z. mays Coleoptile Cells Treated with IAA. Front. Plant Sci. 2019, 9, 01940. [Google Scholar] [CrossRef]
- Kashket, E.R.; Brodie, A.F. Oxidative Phosphorylation in Fractionated Bacterial Systems: X. different roles for the natural quinones of Escherichia coli W in oxidative metabolism. J. Biol. Chem. 1963, 238, 2564–2570. [Google Scholar] [CrossRef]
- Ziegler, D.M. The Role of Quinones in the Mitochondrial Electron Transport System. Am. J. Clin. Nutr. 1961, 9, 43–49. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Mercer, E.I. Introduction to Plant Biochemistry; Pergamon Press: Oxford, UK, 1972. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy secondary metabolites naphthoquinones-their occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 2009, 5, 47–68. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-J.; Chang, F.-C.; Lee, K.-H.; Wang, J.-P.; Teng, C.-M.; Kuo, S.-C. Synthesis and antiplatelet, antiinflammatory, and antiallergic activities of substituted 3-chloro-5,8-dimethoxy-1,4-naphthoquinone and related compounds. Bioorg. Med. Chem. 1998, 6, 2261–2269. [Google Scholar] [CrossRef]
- Jin, Y.-R.; Ryu, C.-K.; Moon, C.-K.; Cho, M.-R.; Yun, Y.-P. Inhibitory effects of J78, a newly synthesized 1,4-naphthoquinone derivative, on experimental thrombosis and platelet aggregation. Pharmacology 2004, 70, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Yuk, D.Y.; Ryu, C.K.; Hong, J.T.; Chung, K.H.; Kang, W.S.; Kim, Y.; Yoo, H.S.; Lee, M.K.; Lee, C.K.; Yun, Y.P. Antithrombotic and antiplatelet activities of 2-chloro-3-[4-(ethylcarboxy)-phenyl]-amino-1,4-naphthoquinone (NQ12), a newly synthesized 1,4-naphthoquinone derivative. Biochem. Pharmacol. 2000, 60, 1001–1008. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Xu, S.; Song, Y. Recent advances of quinones as a privileged structure in drug discovery. Eur. J. Med. Chem. 2021, 223, 113632. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Abe, H.; Yoshizaki, F. In vitro antifungal activity of naphthoquinone derivatives. Biol. Pharm. Bull. 2002, 25, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Vigna, J.; Sighel, D.; Defant, A. Hybrid Molecules Containing Naphthoquinone and Quinolinedione Scaffolds as Antineoplastic Agents. Molecules 2022, 27, 4948. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Valente, C.; Moreira, R.; Guedes, R.C.; Iley, J.; Jaffarc, M.; Douglas, K.T. The 1,4-naphthoquinone scaffold in the design of cysteine protease inhibitors. Bioorg. Med. Chem. 2007, 15, 5340–5350. [Google Scholar] [CrossRef]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Ahmad, Y.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Carnivorous plants as a source of potent bioactive compound: Naphthoquinones. Trop. Plant Biol. 2016, 9, 267–279. [Google Scholar] [CrossRef]
- Johansson, A.C.; Norberg-Spaak, L.; Roberg, K. Role of lysosomal cathepsins in naphthazarin- and Fas-induced apoptosis in oral squamous cell carcinoma cells. Acta Otolaryng. 2006, 126, 70–81. [Google Scholar] [CrossRef]
- Kim, M.Y.; Park, S.J.; Shim, J.W.; Yang, K.; Kang, H.S.; Heo, K. Naphthazarin enhances ionizing radiation-induced cell cycle arrest and apoptosis in human breast cancer cells. Int. J. Oncol. 2015, 46, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Lee, E.K.; Park, S.J.; Kim, N.D.; Hyun, D.-H.; Lee, C.G.; Lee, J.H.; Yang, K.M.; Heo, K.; Son, T.G. Novel anti-cancer role of naphthazarin in human gastric cancer cells. Int. J. Oncol. 2012, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-D.; Li, H.; Li, Y.-C.; Zeng, H. Naphthazarin suppresses cell proliferation and induces apoptosis in human colorectal cancer cells via the B-cell lymphoma 2/B-cell associated X protein signaling pathway. Oncol. Lett. 2016, 12, 5211–5216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monks, T.J.; Highet, R.J.; Lau, S.S. Oxidative cyclization, 1, 4-benzothiazine formation and dimerization of 2-bromo-3-(glutathion-S-yl) hydroquinone. Mol. Pharmacol. 1990, 38, 121–127. [Google Scholar]
- Snell, J.M.; Weissberger, A. The Reaction of Thiol Compounds with Quinones. J. Am. Chem. Soc. 1939, 61, 450–453. [Google Scholar] [CrossRef]
- Micheletti, G.; Boga, C.; Forlani, L.; Del Vecchio, E.; Zanna, N.; Mazzanti, A.; Monari, M. Hydroxy- and Methoxybenzene Derivatives with Benzenediazonium Salts—Chemical Behavior and Tautomeric Problems. Eur. J. Org. Chem. 2016, 2017, 964–974. [Google Scholar] [CrossRef]
- Laugraud, S.; Guingant, A.; Chassagnard, C.; d’Angelo, J. Regioselective Synthesis of 2- and S-(Phenylthio)juglone Derivatives. J. Org. Chem. 1988, 53, 1557–1560. [Google Scholar] [CrossRef]
- Becker, J.; Schuppan, D.; Benzian, H.; Bals, T.; Hahn, E.G.; Cantaluppi, C.; Reichart, P. Immunohistochemical distribution of collagens types IV, V and VI and of pro-collagens types I and III in human alveolar bone and dentine. J. Histochem. Cytochem. 1986, 34, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Chichester, C.O.; Fernandez, M.; Minguell, J.J. Extracellular matrix gene expression by human bone marrow stroma and by marrow fibroblasts. Cell Adhes. Commun. 1993, 1, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Klaus, V.; Hartmann, T.; Gambini, J.; Graf, P.; Stahl, W.; Hartwig, A.; Klotz, L.O. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Arch. Biochem. Biophys. 2010, 496, 93–100. [Google Scholar] [CrossRef]
- Rooseboom, M.; Commandeur, J.N.; Vermeulen, N.P. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004, 56, 53–102. [Google Scholar] [CrossRef]
- Ernster, L. DT Diaphorase: A historical review. Chem. Scr. 1987, 27A, 1–13. [Google Scholar]
- D’Arcy Doherty, M.; Rodgers, A.; Cohen, G.M. Mechanisms of toxicity of 2- and 5-hydroxy-1,4-naphthoquinone; absence of a role for redox cycling in the toxicity of 2-hydroxy-1,4-naphthoquinone to isolated hepatocytes. J. Appl. Toxicol. 1987, 7, 123–129. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Gerber, P.A.; von Montfort, C.; Sies, H.; Klotz, L.O. Epidermal growth factor receptor is a common mediator of quinone-induced signaling leading to phosphorylation of connexin-43—Role of glutathione and tyrosine phosphatases. J. Biol. Chem. 2003, 278, 38360–38367. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Patak, P.; von Montfort, C.; Melchheier, I.; Sies, H.; Klotz, L.O. Signaling effects of menadione: From tyrosine phosphatase inactivation to connexin phosphorylation. Methods Enzymol. 2004, 378, 258–272. [Google Scholar] [CrossRef]
- Micheletti, G.; Calonghi, N.; Farruggia, G.; Strocchi, E.; Palmacci, V.; Telese, D.; Bordoni, S.; Frisco, G.; Boga, C. Synthesis of Novel Structural Hybrids between Aza-Heterocycles and Azelaic Acid Moiety with a Specific Activity on Osteosarcoma Cells. Molecules 2020, 25, 404. [Google Scholar] [CrossRef] [Green Version]










| Starting Quinone | Product (Yield % at 25 °C) a | Product (Yield % at 50 °C) a |
|---|---|---|
| Naphthoquinone (1) | 8 (19) | 8 (34) |
| Menadione (2) | 9 (16) | 9 (25) |
| Plumbagin (3) | 10 (28) | 10 (40) |
| Juglone (4) | 11 + 11a b | 11 + 11a b |
| Naphthazarin (5) | 12 (−) c | 12 (13) d |
| Lawsone (6) | - e | - e |
| Compounds | HeLa IC50 (μM) | SH-SY5Y IC50 (μM) | SaOS2 IC50 (μM) | U2OS IC50 (μM) | HDFa IC50 (μM) |
|---|---|---|---|---|---|
| 8 | 0.54 ± 0.05 | 0.87 ± 0.003 | 1.67 ± 0.01 | 1.81 ± 0.007 | 7.43 ± 0.02 |
| 9 | 0.50 ± 0.08 | 0.96 ± 0.06 | 0.59 ± 0.04 | 0.51 ± 0.06 | 3.87 ± 0.01 |
| 10 | n.a. | n.a. | n.a. | n.a. | n.e. |
| 11 | 13.2 ± 0.05 | 11.4 ± 0.001 | 25.9 ± 0.05 | 30.4 ± 0.005 | 6.88 ± 0.05 |
| 12 | 1.04 ± 0.008 | 0.63 ± 0.04 | 1.56 ± 0.03 | 1.65 ± 0.03 | 0.83 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Boga, C.; Zalambani, C.; Farruggia, G.; Esposito, E.; Fiori, J.; Rizzardi, N.; Taddei, P.; Di Foggia, M.; Calonghi, N. Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity. Molecules 2022, 27, 5645. https://doi.org/10.3390/molecules27175645
Micheletti G, Boga C, Zalambani C, Farruggia G, Esposito E, Fiori J, Rizzardi N, Taddei P, Di Foggia M, Calonghi N. Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity. Molecules. 2022; 27(17):5645. https://doi.org/10.3390/molecules27175645
Chicago/Turabian StyleMicheletti, Gabriele, Carla Boga, Chiara Zalambani, Giovanna Farruggia, Erika Esposito, Jessica Fiori, Nicola Rizzardi, Paola Taddei, Michele Di Foggia, and Natalia Calonghi. 2022. "Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity" Molecules 27, no. 17: 5645. https://doi.org/10.3390/molecules27175645
APA StyleMicheletti, G., Boga, C., Zalambani, C., Farruggia, G., Esposito, E., Fiori, J., Rizzardi, N., Taddei, P., Di Foggia, M., & Calonghi, N. (2022). Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity. Molecules, 27(17), 5645. https://doi.org/10.3390/molecules27175645