Green Synthesis and Characterization of Cobalt Oxide Nanoparticles Using Psidium guajava Leaves Extracts and Their Photocatalytic and Biological Activities
Abstract
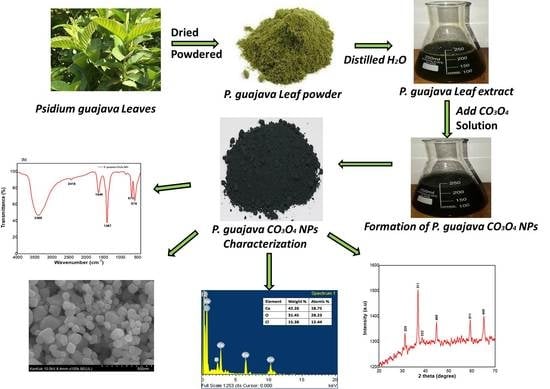
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Analysis
2.1.1. UV-Visible Analysis
2.1.2. XRD Analysis
2.1.3. FTIR Analysis
2.2. Morphological Analysis
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Energy Dispersive X-ray Analysis (EDAX)
2.3. Photocatalytic Activity
2.4. Antioxidant Activity
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.5. Anti-Bacterial Activity
2.6. MTT Assay of Vero, MCF-7 and HCT 116 Cells
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Leaves Extract
3.3. Biosynthesis of Co3O4 NPs
3.4. Characterization
3.5. Photocatalytic Activity
3.6. Antioxidant Activity
3.6.1. DPPH Assay
3.6.2. ABTS Scavenging Activity
3.7. Anti-Bacterial Activity
3.8. Cytotoxicity Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asha, G.; Rajeshwari, V.; Stephen, G.; Gurusamy, S.; Carolin Jeniba Rachel, D. Eco-friendly synthesis and characterization of cobalt oxide nanoparticles by Sativum species and its photo-catalytic activity. Mater. Today Proc. 2022, 48, 486–493. [Google Scholar] [CrossRef]
- Govindasamy, R.; Govindarasu, M.; Alharthi, S.S.; Mani, P.; Bernaurdshaw, N.; Gomathi, T.; Ansari, M.A.; Alomary, M.N.; Atwah, B.; Malik, M.S.; et al. Sustainable green synthesis of yttrium oxide (Y2O3) nanoparticles using Lantana camara leaf extracts: Physicochemical characterization, photocatalytic degradation, antibacterial, and anticancer potency. Nanomaterials 2022, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Buledi, J.A.; Amin, S.; Haider, S.I. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2021, 28, 58994–59002. [Google Scholar] [CrossRef] [PubMed]
- Mehmeti, E.; Stankovic, D.M.; Chaiyo, S.; Svorc, L.; Kalcher, K. Manganese dioxide-modified carbon paste electrode for voltammetric determination of riboflavin. Microchim. Acta 2016, 183, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2/Co3O4/reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Fayemi, O.; Adekunle, A. Metal oxide nanoparticles/multi-walled carbon nanotube nanocomposite modified electrode for the detection of dopamine: Comparative electrochemical study. J. Biosens. Bioelectron. 2015, 6, 190. [Google Scholar] [CrossRef]
- Maeda, K.; Ishimaki, K.; Tokunaga, Y.; Lu, D.; Eguchi, M. Modification of wide-band-gap oxide semiconductors with cobalt hydroxide nanoclusters for visible-light water oxidation. Angew. Chem. Int. 2016, 5, 8309–8313. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Alamry, K.A. Green synthesis of 3D hierarchical nanostructured Co3O4/carbon catalysts for the application in sodium borohydride hydrolysis. J. Alloys Compd. 2019, 798, 820–831. [Google Scholar] [CrossRef]
- Ledo-Suárez, A.; Rodríguez-Sánchez, L.; Blanco, M.C.; López-Quintela, M.A. Electrochemical synthesis and stabilization of cobalt nanoparticles. Phys. Stat. Sol. 2006, 203, 1234–1240. [Google Scholar] [CrossRef]
- Alex, P.; Majumdar, S.; Kishor, J.; Sharma, I.G. Synthesis of cobalt nano crystals in aqueous media and its characterization. Mater. Sci. Appl. 2011, 2, 1307–1312. [Google Scholar] [CrossRef]
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene doping: A review. Insci. J. 2011, 1, 80. [Google Scholar] [CrossRef]
- Mindru, I.; Gingasu, D.; Patron, L.; Ianculescu, A.; Surdu, V.-A.; Culita, D.C.; Preda, S.; Negut, C.-D.; Oprea, O. A new approach: Synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater. Sci. Eng. B 2019, 246, 42–48. [Google Scholar] [CrossRef]
- Ma, J.; Manthiram, A. Precursor-directed formation of hollow Co3O4 nanospheres exhibiting superior lithium storage properties. RSC Adv. 2012, 2, 3187–3189. [Google Scholar] [CrossRef]
- Muneer, M.; Mumtaz, M.; Siddique, N.; Akram, M.; Hamayun, M. Ag-Co3O4: Synthesis, characterization and evaluation of its photo-catalytic activity towards degradation of rhodamine B dye in aqueous medium. Chin. J. Chem. Eng. 2018, 26, 1264–1269. [Google Scholar]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–8672. [Google Scholar] [CrossRef]
- Shinde, V.; Mahadik, S.; Gujar, T.; Lokhande, C. Super capacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 2006, 252, 7487–7492. [Google Scholar] [CrossRef]
- Smith, G.B.; Ignatiev, A.; Zajac, G. Solar selective black cobalt: Preparation structure, and thermal stability. J. Appl. Phys. 1980, 51, 4186–4196. [Google Scholar] [CrossRef]
- Sorbiun, M.; Mehr, E.; Ramazani, A.; Malekzadeh, A. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their anti-bacterial properties. Nanochem. Res. 2018, 3, 1–16. [Google Scholar]
- Thomas, M.; Naikoo, G.A.; Sheikh, M.U.D.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A Chem. 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Jadhav, J.; Phugare, S.; Dhanve, R.; Jadhav, S. Rapid biodegradation and decolourization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain. BCH Biodegrad. 2010, 21, 453–463. [Google Scholar] [CrossRef]
- Ljubas, D.; Smoljanić, G.; Juretić, H. Degradation of Methyl Orange and Congo Red dyes by using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manag. 2015, 161, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Szpyrkowicz, L. Hydrodynamic effects on the performance of electrocoagulation/electro-flotation for the removal of dyes from textile wastewater. Ind. Eng. Chem. Res. 2005, 44, 7844–7853. [Google Scholar] [CrossRef]
- Kim, Y.; Hensley, R. Effective control of chlorination and dechlorination at wastewater treatment plants using redox potential. Water Environ. Res. 1997, 69, 1008–1014. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Kandpal, N.D.; Sah, N.; Loshali, R.; Joshi, R.; Prasad, J. Co-precipitation method of synthesis and characterization of iron oxide nanoparticles. J. Sci. Ind. Res. 2014, 73, 87–90. [Google Scholar]
- Bayal, N.; Jeevanandam, P. Synthesis of TiO2−MgO mixed metal oxide nanoparticles via a sol−gel method and studies on their optical properties. Ceram. Int. 2014, 40, 15463–15477. [Google Scholar] [CrossRef]
- Salimi, A.; Mamkhezri, H.; Hallaj, R.; Soltanian, S. Electrochemical detection of trace amount of arsenic(III) at glassy carbon electrode modified with cobalt oxide nanoparticles. Sens. Actuators B Chem. 2008, 129, 246–254. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Thomas, A. A Generalized synthesis of metal oxide hollow spheres using a hydrothermal approach. Chem. Mater. 2006, 18, 3808–3812. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Idhayadhulla, A.; Lee, Y.R. Preparation of Au and Ag nanoparticles using Artemisia annua and them in vitro anti-bacterial and tyrosinase inhibitory activities. Mater. Sci. Eng. C 2014, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.H.; Khan, A.; Chen, Y.; Shah, N.S.; Muhammad, N.; Khan, A.U.; Tahir, K.; Khan, F.U.; Murtaza, B.; Hassan, S.U.; et al. Biomedical applications of green synthesized Nobel metal nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 173, 150–164. [Google Scholar] [CrossRef]
- Dubey, S.; Kumar, J.; Kumar, A.; Sharma, Y.C. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: Characterization and screening of its eco-toxicity. Adv. Powder Technol. 2018, 29, 2583–2590. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S.; Safa, Y.; Jilani, K.; Sultan, M.; Ata, S.; et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Dakappa, S.S.; Adhikari, R.; Timilsina, S.S.; Sajjekhan, S. A review on the medicinal plant Psidium guajava Linn. (Myrtaceae). J. Drug Deliv. Ther. 2013, 3, 162–168. [Google Scholar] [CrossRef]
- Lozoya, X.; Meckes, M.; Abou-Zaid, M.; Tortoriello, J.; Nozzolillo, C.; Arnason, J.T. Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Arch. Med. Res. 1994, 25, 11–15. [Google Scholar]
- Murray, M.T.; Pizzorno, J.E. Flavonoids-Quercetin, citrus favonoids, and HERs (hydroxylethylrutosides). In Text Book of Natural Medicine, 2nd ed.; Pizzorno, J.E., Murray, M.E., Eds.; Harcourt Brace and Company Ltd.: London, UK, 1999; pp. 745–750. [Google Scholar]
- Ojewole, J.A. Anti-Inflammatory and analgesic effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extracts in rats and mice. Methods Find Exp. Clin. Pharmacol. 2006, 28, 441–446. [Google Scholar] [CrossRef]
- Nair, R.; Chanda, S. In-vitro antimicrobial activity of Psidium guajava L. leaf extracts against clinically important pathogenic microbial strains. Braz. J. Microbiol. 2007, 38, 452–458. [Google Scholar] [CrossRef]
- Roy, K.; Kamath, V.; Asad, M. Hepatoprotective activity of Psidium guajava L. leaf extract. Indian J. Exp. Biol. 2006, 44, 305–311. [Google Scholar] [PubMed]
- Chen, H.-Y.; Yen, G.-C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007, 101, 686–694. [Google Scholar] [CrossRef]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Abraham, N. Effect of added rosemary extract on oxidative stability of Camelina sativa oil. Acta Agric. Slov. 2006, 87, 255–261. [Google Scholar]
- Anosike, C.A.; Obido, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharma. Sci. 2012, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pant, K.; Agarwal, K.; Saini, P. To study in vitro anti-inflammatory activity of Anthracephalus cadamba leaves extract. DHR Int. J. Pharma. Sci. 2012, 3, 55–60. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: New York, NY, USA, 1995. [Google Scholar]
- Lisiecki, I.; Pileni, M.P. Synthesis of well-defined and low size distribution cobalt nanocrystals: The limited influence of reverse micelles. Langmuir 2003, 19, 9486–9489. [Google Scholar] [CrossRef]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its anti-bacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef]
- Shah, G.M.; Khan, M.A. Common medicinal folk recipes of Siran Valley, Mansehra, Pakistan Ethnobot. Leaflets 2008, 10, 49–62. [Google Scholar]
- Ahmad, N.; Sharma, S.; Rai, R. Rapid green synthesis of silver and gold nanoparticles using peels of Punica granatum. Adv. Mater. Lett. 2012, 3, 376–380. [Google Scholar] [CrossRef]
- Nazeruddin, G.; Prasad, N.; Prasad, S.; Garadkar, K.; Nayak, A.K. In-vitro biofabrication of silver nanoparticle using Adhathoda vasica leaf extract and its anti-microbial activity. Phys. E Low-Dimen. Syst. Nanostruct. 2014, 61, 56–61. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, characterization, and properties of metallic copper nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- Athar, T.; Hakeem, A.; Topnani, N.; Hashmi, A. Wet synthesis of monodisperse cobalt oxide nanoparticles. ISRN Mater. Sci. 2012, 2012, 691032. [Google Scholar] [CrossRef]
- Iqbal, M.; Nisar, J.; Adil, M.; Abbas, M.; Riaz, M.; Tahir, M.A.; Younus, M.; Shahid, M. Mutagenicity and cytotoxicity evaluation of photo-catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere 2017, 168, 590–598. [Google Scholar] [CrossRef]
- Ashar, A.; Iqbal, M.; Bhatti, I.A.; Ahmad, M.Z.; Qureshi, K.; Nisar, J.; Bukhari, I.H. Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: Nonylphenol ethoxylate degradation under UV and solar irradiation. J. Alloys Compd. 2016, 678, 126–136. [Google Scholar] [CrossRef]
- Iqbal, M.; Bhatti, I.A. Gamma radiation/H2O2 treatment of a nonylphenol ethoxylates: Degradation, cytotoxicity, and mutagenicity evaluation. J. Hazard. Mater. 2015, 299, 351–360. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium guajava L.) Leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 2021, 10, 752. [Google Scholar] [CrossRef]
- Baumann, J.; Wurn, G.; Bruchlausen, F.V. Prostaglandin synthetase inhibiting O2 radical scavenging properties of some flavonoids and related phenolic compounds. Deutsche Pharmakologische Gesellschaft abstracts of the 20th spring meeting. Arc. Pharmacol. 1979, 307, R1–R77. [Google Scholar]
- Jahani, M.; Khavari-Nejad, R.A.; Mahmoodzadeh, H.; Saadatmand, S. Effects of cobalt oxide nanoparticles (Co3O4 NPs) on ion leakage, total phenol, antioxidant enzymes activities and cobalt accumulation in Brassica napus L. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1260–1275. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, E.; Ndebia, E.J.; Meeme, A.; Adam, B.; Menziwa, P.; Nkeh-Chungag, B.N.; Iputo, J.E. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 2010, 4, 789–795. [Google Scholar]
- Okoli, C.O.; Akah, P.A.; Onuoha, N.J.; Okoye, T.C.; Nwoye, A.C.; Nworu, C.S. Acanthus montanus: An experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement. Altern. Med. 2008, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Wahab, R.; Ahmad, J.; Farshori, N.N.; Al-Khedhairy, A.A. Single and multi-metal oxide nanoparticles induced cytotoxicity and ROS generation in human breast cancer (MCF-7) Cells. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4106–4116. [Google Scholar] [CrossRef]
- Gaikar, P.S.; Angre, A.P.; Wadhawa, G.; Ledade, P.V.; Mahmood, S.H.; Lambat, T.L. Green synthesis of cobalt oxide thin films as an electrode material for electrochemical capacitor application. Curr. Res. Green Sustain. Chem. 2022, 5, 100265. [Google Scholar] [CrossRef]
- Govindarasu, M.; Abirami, P.; Rajakumar, G.; Ansari, M.A.; Alomary, M.N.; Alkhayl, F.F.A.; Aloliqi, A.A.; Thiruvengadam, M.; Vaiyapuri, M. Kaempferitrin inhibits colorectal cancer cells by inducing reactive oxygen species and modulating PI3K/AKT signalling pathway. Process Biochem. 2022, 116, 26–37. [Google Scholar] [CrossRef]












| Peak Position 2θ (Degree) | FWHM β (Degree) | Crystallite Size (nm) |
|---|---|---|
| 32.35 | 0.321 | 26.92 |
| 36.69 | 0.373 | 23.45 |
| 39.24 | 0.268 | 32.28 |
| 44.76 | 0.241 | 37.25 |
| 59.42 | 0.315 | 30.24 |
| 67.35 | 0.282 | 35.38 |
| Average crystallite size (nm) | 30.92 nm |
| Concentration (µg/mL) | Zone of Inhibition (mm) S. aureus | E. coli |
|---|---|---|
| 50 | 9 ± 1.25 | 7 ± 0.95 |
| 100 | 12 ± 1.97 | 10 ± 1.83 |
| 150 | 16 ± 2.45 | 13 ± 2.08 |
| 200 | 18 ± 2.69 | 15 ± 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindasamy, R.; Raja, V.; Singh, S.; Govindarasu, M.; Sabura, S.; Rekha, K.; Rajeswari, V.D.; Alharthi, S.S.; Vaiyapuri, M.; Sudarmani, R.; et al. Green Synthesis and Characterization of Cobalt Oxide Nanoparticles Using Psidium guajava Leaves Extracts and Their Photocatalytic and Biological Activities. Molecules 2022, 27, 5646. https://doi.org/10.3390/molecules27175646
Govindasamy R, Raja V, Singh S, Govindarasu M, Sabura S, Rekha K, Rajeswari VD, Alharthi SS, Vaiyapuri M, Sudarmani R, et al. Green Synthesis and Characterization of Cobalt Oxide Nanoparticles Using Psidium guajava Leaves Extracts and Their Photocatalytic and Biological Activities. Molecules. 2022; 27(17):5646. https://doi.org/10.3390/molecules27175646
Chicago/Turabian StyleGovindasamy, Rajakumar, Vaishnavi Raja, Sonalika Singh, Mydhili Govindarasu, Sulthana Sabura, Kaliaperumal Rekha, V. Devi Rajeswari, Salman S. Alharthi, Manju Vaiyapuri, Rajagopal Sudarmani, and et al. 2022. "Green Synthesis and Characterization of Cobalt Oxide Nanoparticles Using Psidium guajava Leaves Extracts and Their Photocatalytic and Biological Activities" Molecules 27, no. 17: 5646. https://doi.org/10.3390/molecules27175646
APA StyleGovindasamy, R., Raja, V., Singh, S., Govindarasu, M., Sabura, S., Rekha, K., Rajeswari, V. D., Alharthi, S. S., Vaiyapuri, M., Sudarmani, R., Jesurani, S., Venkidasamy, B., & Thiruvengadam, M. (2022). Green Synthesis and Characterization of Cobalt Oxide Nanoparticles Using Psidium guajava Leaves Extracts and Their Photocatalytic and Biological Activities. Molecules, 27(17), 5646. https://doi.org/10.3390/molecules27175646