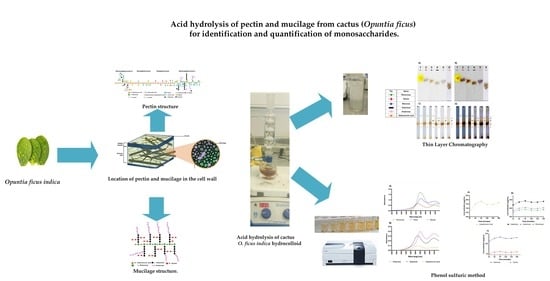
Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Acid Hydrolysis of O. ficus-indica Hydrocolloids
3.2.2. Thin-Layer Chromatography
3.2.3. Phenol–Sulfuric Acid Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Torrero, E.; Garcia-Tovar, S.E.; Luna-Rodriguez, L.E.; RodríguezGarcia, M.E. Chemical Composition of Prickly Pads from Opuntia ficus-indica (L.) Miller Related to Maturity Stage and Environment. Int. J. Plant Biol. Res. 2017, 5, 1–7. [Google Scholar]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and Isolation of Anti-Pneumonia Bioactive Compounds from Opuntia ficus-indica Fruit Waste Peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; Valderrama-Bravo, M.D.C.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and Rheological Characterization of Opuntia Ficus Mucilage at Three Different Maturity Stages of Cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Amin, E.S.; Awad, O.M.; El-Sayed, M. The Mucilage of Opuntia ficus-indica Mill. Carbohydr. Res. 1970, 15, 159–161. [Google Scholar] [CrossRef]
- McGarvie, D.; Parolis, H. Methylation Analysis of the Mucilage of Opuntia ficus-indica. Carbohydr. Res. 1981, 88, 305–314. [Google Scholar] [CrossRef]
- Tripodo, M.; Lanuzza, F.; Mondello, F.; Occhiuto, F.; Galati, E. Enzymatic Extraction of Pectin from Opuntia ficus-indica Cladodes. In VIII International Congress on Cactus Pear and Cochineal 1067; ISHS: Palermo, Italy, 2013. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic Extraction of Pectin from Opuntia Ficus Indica Cladodes After Mucilage Removal: Optimization of Experimental Conditions and Evaluation of Chemical and Functional Properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- McGarvie, D.; Parolis, H. The Mucilage of Opuntia ficus-indica. Carbohydr. Res. 1979, 69, 171–179. [Google Scholar] [CrossRef]
- Haughn, G.W.; Western, T.L. Arabidopsis Seed Coat Mucilage is a Specialized Cell Wall that Can be Used as a Model for Genetic Analysis of Plant Cell Wall Structure and Function. Front. Plant Sci. 2012, 3, 64. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Ornelas-Paz, J.D.J.; Martínez-Téllez, M.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.; Hugo, A. Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules 2018, 23, 916. [Google Scholar] [CrossRef] [Green Version]
- Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Cárdenas, A. Pectins from Opuntia spp.: A Short Review. J. Prof. Assoc. Cactus Dev. 2003, 5, 17–29. [Google Scholar]
- Nobel, P.S.; Cavelier, J.; Andrade, J.L. Mucilage in Cacti: Its Apoplastic Capacitance, Associated Solutes, and Influence on Tissue 5. J. Exp. Bot. 1992, 43, 641–648. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Chichester, West Sussex, 2015. [Google Scholar]
- BeMiller, J.N. Carbohydrate Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 147–177. [Google Scholar]
- Caffall, K.H.; Mohnen, D. The Structure, Function, And Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Tanczos, I.; Schwarzinger, C.; Schmidt, H.; Balla, J. THM-GC/MS Analysis of Model Uronic Acids of Pectin and Hemicelluloses. J. Anal. Appl. Pyrolysis 2003, 68, 151–162. [Google Scholar] [CrossRef]
- Cárdenas, Y.; Ríos-Silva, M.; Huerta, M.; López, M.; Bricio-Barrios, J.; Ortiz-Mesina, M.; Urzúa, Z.; Saavedra-Molina, A.; Trujillo, X. The Comparative Effect of Nopal and Mucilage in Metabolic Parameters in Rats with a High-Fructose Diet. J. Med. Food 2019, 22, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Burton, R.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the Chemistry, Structure and Function of Plant Cell Walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.K. Natural Polymers, Gums and Mucilages as Excipients in Drug Delivery. Polim. Med. 2012, 42, 191–197. [Google Scholar] [PubMed]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef]
- Du Toit, A. Selection, Extraction, Characterization and Application of Mucilage from Cactus Pear (Opuntia Ficus-Indica and Opuntia Robusta) Cladodes; University of the Free State: Bloemfontein, South Africa, 2016. [Google Scholar]
- Saag, L.M.K.; Sanderson, G.R.; Moyna, P.; Ramos, G. Cactaceae Mucilage Composition. J. Sci. Food Agric. 1975, 26, 993–1000. [Google Scholar] [CrossRef]
- León-Martínez, F.; Méndez-Lagunas, L.; Rodríguez-Ramírez, J. Spray Drying of Nopal Mucilage (Opuntia Ficus-Indica): Effects on Powder Properties and Characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Bala, R.; Rana, R.; Madaan, R. Natural Gums and Mucilage as Matrix Formers in Sustained Released Dosage Forms. Res. J. Pharm. Technol. 2019, 12, 5119–5125. [Google Scholar] [CrossRef]
- Rodríguez-Garcia, M.E.; De Lira, C.; Hernández-Becerra, E.; Villegas, M.D.L.A.C.; Palacios, A.; Rojas-Molina, I.; Reynoso, R.; Quintero, L.C.; Del-Real, A.; Zepeda, T.A.; et al. Physicochemical Characterization of Nopal Pads (Opuntia ficus indica) and Dry Vacuum Nopal Powders as a Function of the Maturation. Mater. Veg. 2007, 62, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Chapter 1—Type-2 Diabetes: Definition, Diagnosis and Significance. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Habtemariam, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–10. [Google Scholar] [CrossRef]
- Guevara-Arauza, J.C.; Ornelas-Paz, J.D.J.; Pimentel, D.; Mendoza, S.R.; Guerra, R.E.S.; Maldonado, L.M.T.P. Prebiotic Effect of Mucilage and Pectic-Derived Oligosaccharides from Nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and Characterization of Mucilage in Opuntia spp. J. Arid. Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp Mucilage’s: A Functional Component with Industrial Perspectives. J. Arid Environ. 2003, 57, 275–290. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Farias, D.D.P.; Neri-Numa, I.A.; Pastore, G.M. Underutilized Plants of the Cactaceae Family: Nutritional Aspects and Technological Applications. Food Chem. 2021, 362, 130196. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant Seed Mucilage as Emerging Biopolymer in Food Industry Applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of Yellow-Orange Cactus Pear Pulp (Opuntia ficus-indica) with Cladode Mucilage and Maltodextrin as a Food Coloring in Yogurt. LWT 2020, 138, 110672. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Romo, M.G.G.; Chacón-García, L. Extraction and Characterization of Mucilage from Wild Species of O puntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Sandoval, D.C.G.; Sosa, B.L.; Martínez-Ávila, G.C.G.; Fuentes, H.R.; Abarca, V.H.A.; Rojas, R. Formulation and Characterization of Edible Films Based on Organic Mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of Nopal Mucilage Addition on Physical, Barrier and Mechanical Properties of Citric Pectin-Based Films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Badui Dergal, S. Química de Los Alimentos; Pearson Educación: Naucalpan de Juarez, Mexico, 2006. [Google Scholar]
- Waldron, K. Handbook of Waste Management and Co-Product Recovery in Food Processing; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Shi, H.; Wan, Y.; Li, O.; Zhang, X.; Xie, M.; Nie, S.; Yin, J. Two-Step Hydrolysis Method for Monosaccharide Composition Analysis of Natural Polysaccharides Rich in Uronic Acids. Food Hydrocoll. 2020, 101, 105524. [Google Scholar] [CrossRef]
- UÇAR, G.; Balaban, M. Hydrolysis of Polysaccharides with 77% Sulfuric Acid for Quantitative Saccharification. Turk. J. Agric. For. 2003, 27, 361–365. [Google Scholar]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide Composition Analysis of Polysaccharides from Natural Sources: Hydrolysis Condition and Detection Method Development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Huang, G. Extraction and Analysis of Polysaccharide from Momordica Charantia. Ind. Crop. Prod. 2020, 153, 112588. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Z.; Linhardt, R.J. Thin Layer Chromatography for the Separation and Analysis of Acidic Carbohydrates. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1711–1732. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. Thin Layer Chromatography of Carbohydrates. Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 251–253. [Google Scholar] [CrossRef]
- Chaplin, M.; Kennedy, J.J.M.S. Monosaccharides; University of Oxford: Oxford, UK, 1986; Volume 1, p. 7. [Google Scholar]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An Improved Phenol-Sulfuric Acid Method for the Determination of Carbohydrates in the Presence of Persulfate. Carbohydr. Polym. 2019, 227, 115332. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F.; Shah, M.A. Analysis of Carbohydrates (Monosaccharides, Polysaccharides). Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 621–633. [Google Scholar] [CrossRef]
- Wolfgong, W.J. Chemical Analysis Techniques for Failure Analysis: Part 1, Common Instrumental Methods. Handbook of Materials Failure Analysis with Case Studies from the Aerospace and Automotive Industries; Elsevier: Amsterdam, The Netherlands, 2016; pp. 279–307. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Meyrand, M.; German, J.B.; Lebrilla, C.B.; Coisson, J.D.; Arlorio, M.; Barile, D. Identification and Characterization of Complex Bioactive Oligosaccharides in White and Red Wine by a Combination of Mass Spectrometry and Gas Chromatography. J. Agric. Food Chem. 2012, 60, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-B.; Yang, X.; Wang, J.; Zhao, H.-T.; Lu, W.-H.; Cui, J.; Cheng, C.-L.; Zou, P.; Huang, W.-W.; Wang, P.; et al. Chemical Composition and Antioxidant Activities of Three Polysaccharide Fractions from Pinecones. Int. J. Mol. Sci. 2012, 13, 14262–14277. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, W.-J.; Wang, W.; Sun, M.-X.; Feng, Y.-Q. Rapid Analysis of Monosaccharides in Sub-milligram Plant Samples Using Liquid Chromatography–Mass Spectrometry Assisted by Post-column Derivatization. J. Agric. Food Chem. 2020, 68, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, D.; Manfredini, S.; Vertuani, S.; Bernardi, T. New Insights into Sugar Characterization in Complex Plant Matrices by High-Performance Thin-Layer Chromatography. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 607–612. [Google Scholar] [CrossRef]
- Zhang, Z.; Khan, N.M.; Nunez, K.M.; Chess, E.K.; Szabo, C.M. Complete Monosaccharide Analysis by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection. Anal. Chem. 2012, 84, 4104–4110. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Ren, W.-C.; Su, Y.; Li, J.; Du, Y.-Q.; Wang, Q.-H.; Kuang, H.-X. Rapid Determination and Origin Identification of Total Polysaccharides Contents in Schisandra Chinensis by Near-Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120327. [Google Scholar] [CrossRef] [PubMed]
- Garna, H.; Mabon, N.; Nott, K.; Wathelet, B.; Paquot, M. Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chem. 2006, 96, 477–484. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Curto, R.B.L.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntia ficus indica Fruit Gum: Extraction, Characterization, Antioxidant Activity and Functional Properties. Carbohydr. Polym. 2018, 206, 565–572. [Google Scholar] [CrossRef]
- Reyes-Ocampo, I.; Córdova-Aguilar, M.S.; Guzmán, G.; Blancas-Cabrera, A.; Ascanio, G. Solvent-Free Mechanical Extraction of Opuntia ficus-indica mucilage. J. Food Process Eng. 2018, 42, e12954. [Google Scholar] [CrossRef]
- Texco, A.; De Pachuca, U.P. Optimization of the Acid Hydrolysis of Cladodes of Opuntia Ficus-Indica by Response Surface Methodology. Rev. Mex. De Ing. Química 2018, 17, 1095–1104. [Google Scholar] [CrossRef]
- Santagata, G.; Cimmino, A.; Poggetto, G.D.; Zannini, D.; Masi, M.; Emendato, A.; Surico, G.; Evidente, A. Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties. Biomolecules 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]







| Galacturonic Acid | Rhamnose | Galactose | Arabinose | Xylose | Mannose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM | |
| 0.4 | 0.006 | 0.47 | 0.015 | 0.39 | 0.044 | 0.40 | 0.009 | 0.43 | 0.013 | 0.4 | 0.009 | |
| Galacturonic Acid | Rhamnose | Galactose | Arabinose | Xylose | Mannose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM | Mean | SEAM |
| 30 | 650.71 | 18.89 | 155.58 | 4.47 | 240.27 | 6.20 | 173.70 | 5.07 | 50.49 | 1.49 | 111.41 | 2.89 |
| 60 | 753.95 | 31.65 | 180.00 | 7.49 | 273.78 | 9.16 | 201.40 | 8.49 | 58.66 | 2.50 | 127.01 | 4.27 |
| 90 | 784.98 | 29.59 | 187.34 | 7.00 | 287.54 | 10.35 | 209.73 | 7.94 | 61.11 | 2.34 | 133.42 | 4.82 |
| 120 | 743.96 | 26.74 | 177.64 | 6.32 | 272.32 | 9.13 | 198.72 | 7.17 | 57.87 | 2.12 | 126.33 | 4.25 |
| 150 | 745.38 | 26.47 | 177.97 | 6.26 | 273.49 | 9.10 | 199.10 | 7.10 | 57.98 | 2.10 | 126.88 | 4.24 |
| 180 | 767.76 | 11.20 | 183.27 | 2.65 | 282.13 | 3.39 | 205.11 | 3.00 | 59.75 | 0.89 | 130.90 | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garfias Silva, V.; Cordova Aguilar, M.S.; Ascanio, G.; Aguayo, J.P.; Pérez-Salas, K.Y.; Susunaga Notario, A.d.C. Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides. Molecules 2022, 27, 5830. https://doi.org/10.3390/molecules27185830
Garfias Silva V, Cordova Aguilar MS, Ascanio G, Aguayo JP, Pérez-Salas KY, Susunaga Notario AdC. Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides. Molecules. 2022; 27(18):5830. https://doi.org/10.3390/molecules27185830
Chicago/Turabian StyleGarfias Silva, Vanessa, María Soledad Cordova Aguilar, Gabriel Ascanio, Juan Pablo Aguayo, Karen Y. Pérez-Salas, and Ana del Carmen Susunaga Notario. 2022. "Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides" Molecules 27, no. 18: 5830. https://doi.org/10.3390/molecules27185830
APA StyleGarfias Silva, V., Cordova Aguilar, M. S., Ascanio, G., Aguayo, J. P., Pérez-Salas, K. Y., & Susunaga Notario, A. d. C. (2022). Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides. Molecules, 27(18), 5830. https://doi.org/10.3390/molecules27185830