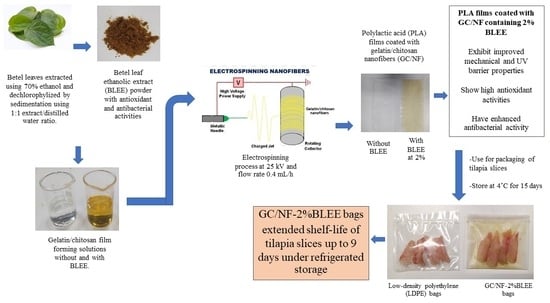
Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of PLA Films Coated with Electrospun GC/NF with, and without, BLEE at Different Concentrations
2.1.1. Morphology
2.1.2. Thickness
2.1.3. Mechanical Properties
2.1.4. Water Vapor Permeability (WVP)
2.1.5. Oxygen Permeability (OP)
2.1.6. Color, Light Transmission, and Transparency
2.1.7. Bioactivities of PLA Films Coated with Electrospun GC/NF without and with BLEE at Different Concentrations
Antioxidant Activities (AO-A)
Antibacterial Activities (AB-A)
2.1.8. Thermal Stability
2.2. Quality Changes of Nile Tilapia Slices during Refrigerated Storage
2.2.1. Changes in Microbiological Load
2.2.2. Changes in Chemical Indices
3. Materials and Methods
3.1. Materials
3.2. Preparation and Dechlorophyllization of Betel Leaf Ethanolic Extracts (BLEE)
3.3. Preparation of Polylactic Acid (PLA) Film
3.4. Preparation of Electrospun Gelatin/Chitosan Nanofiber (GC/NF) Incorporated with BLEE
3.5. Characterization of PLA Films Coated with Electrospun GC/NF without and with BLEE at Various Concentrations
3.5.1. Morphology
3.5.2. Thickness
3.5.3. Mechanical Properties
3.5.4. Water Vapor Permeability (WVP)
3.5.5. Oxygen Permeability (OP)
3.5.6. Color, Light Transmission, and Transparency
3.5.7. Bioactivities of PLA Films Coated with Electrospun GC/NF without and with BLEE at Various Concentrations
Antioxidant Activities (AO-A)
Antibacterial Activity (AB-A)
3.5.8. Thermogravimetric Analysis (TGA)
3.6. Quality Changes in Nile Tilapia Slices Packaged in Bags Prepared from the Selected PLA Films Coated with GC/NF Containing BLEE during Refrigerated Storage
3.6.1. Preparation of Nile Tilapia slices
3.6.2. Preparation of Bags
3.6.3. Microbiological Analyses
3.6.4. Chemical Analyses
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/thermal properties and heat seal ability of bilayer films based on fish gelatin and poly (lactic acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Fonseca, L.M.; dos Santos Cruxen, C.E.; Bruni, G.P.; Fiorentini, Â.M.; da Rosa Zavareze, E.; Lim, L.-T.; Guerra Dias, A.R. Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int. J. Biol. Macromol. 2019, 139, 1182–1190. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.-H.; Chiu, F.-C. Manufacturing poly (butylene adipate-co-terephthalate)/high density polyethylene blend-based nanocomposites with enhanced burning anti-dripping and physical properties—Effects of carbon nanofillers addition. Compos. Part. B Eng. 2021, 217, 108878. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of biodegradable PLA nonwoven fabric as superoleophilic and superhydrophobic material for efficient oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Kaveh, F.; Schmid, M. Facile fabrication of transparent high-barrier poly (lactic acid)-based bilayer films with antioxidant/antimicrobial performances. Food Chem. 2022, 384, 132540. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Sun, Z.; Wang, D.; Wu, H.; Du, L.; Wang, D. Preparation and antibacterial properties of ε-polylysine-containing gelatin/chitosan nanofiber films. Int. J. Biol. Macromol. 2020, 164, 3376–3387. [Google Scholar] [CrossRef]
- Morie, A.; Garg, T.; Goyal, A.K.; Rath, G. Nanofibers as novel drug carrier—An overview. Artif. Cells Nanomed. Biotechnol. 2016, 44, 135–143. [Google Scholar] [CrossRef]
- Giménez, B.; De Lacey, A.L.; Pérez-Santín, E.; López-Caballero, M.; Montero, P. Release of active compounds from agar and agar–gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S. Ethanolic extract of Betel (Piper betle L.) and Chaphlu (Piper sarmentosum Roxb.) dechlorophyllized using sedimentation process: Production, characteristics, and antioxidant activities. J. Food Biochem. 2020, 44, e13508. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Ahmed, S.; Qin, W.; Liu, Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag. Shelf Life 2019, 22, 100390. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Fathi, M.; Kadivar, M. Production and characterization of chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to enhance edible film’s properties. Food Packag. Shelf Life 2019, 22, 100387. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control. 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Busolo, M.; Cherpinski, A.; Lagaron, J. Electrospinning in the packaging industry. Electrospinning 2018, 238–260. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Electrospinning of gelatin/chitosan nanofibers incorporated with tannic acid and chitooligosaccharides on polylactic acid film: Characteristics and bioactivities. Food Hydrocoll. 2022, 133, 107916. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part. C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Zhan, P.; Dai, K.; Zheng, G.; Liu, C.; Shen, C. A tunable strain sensor based on a carbon nanotubes/electrospun polyamide 6 conductive nanofibrous network embedded into poly (vinyl alcohol) with self-diagnosis capabilities. J. Mater. Chem. C 2017, 5, 4408–4418. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Kester, J.; Fennema, O. An edible film of lipids and cellulose ethers: Barrier properties to moisture vapor transmission and structural evaluation. J. Food Sci. 1989, 54, 1383–1389. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: Effect of type of polymer matrix. LWT 2016, 74, 338–345. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Properties of fish skin gelatin film incorporated with seaweed extract. J. Food Eng. 2009, 95, 151–157. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 2019, 138, 111578. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.S.; Choi, J.H.; Jeong, C.M.; Sung, M.H.; Park, H.J. Improvements in barrier properties of poly (lactic acid) films coated with chitosan or chitosan/clay nanocomposite. J. Appl. Polym. Sci. 2012, 125, E675–E680. [Google Scholar] [CrossRef]
- Sobral, P.; Habitante, A. Phase transitions of pigskin gelatin. Food Hydrocoll. 2001, 15, 377–382. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Suite, NW, USA, 2007; p. 195, ISBN-13: 978-1-4200-2052-6. [Google Scholar]
- de Campos, S.S.; de Oliveira, A.; Moreira, T.F.M.; da Silva, T.B.V.; da Silva, M.V.; Pinto, J.A.; Bilck, A.P.; Gonçalves, O.H.; Fernandes, I.P.; Barreiro, M.-F.; et al. TPCS/PBAT blown extruded films added with curcumin as a technological approach for active packaging materials. Food Packag. Shelf Life 2019, 22, 100424. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S.; Zhang, B. Use of betel leaf (Piper betle L.) ethanolic extract in combination with modified atmospheric packaging and nonthermal plasma for shelf-life extension of Nile tilapia (Oreochromis niloticus) fillets. J. Food Sci. 2021, 86, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and antioxidant properties of chitosan polymers obtained from nontraditional Polybius henslowii sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Benjakul, S.; Prodpran, T.; Nilsuwan, K.; Huda, N.; de la Caba, K. Composite films based on chitosan and epigallocatechin gallate grafted chitosan: Characterization, antioxidant and antimicrobial activities. Food Hydrocoll. 2021, 111, 106384. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Nouri, L.; Nafchi, A.M. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Laaziz, S.A.; Raji, M.; Hilali, E.; Essabir, H.; Rodrigue, D.; Bouhfid, R. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, H.L.; Bendaikha, H.; Maschke, U. Green composites based on Atriplex halimus fibers and PLA matrix. J. Polym. Eng. 2020, 40, 693–702. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined Effect of ethanolic coconut husk extract and modified atmospheric packaging (MAP) in extending the shelf life of Asian sea bass slices. J. Aquat. Food Prod. Technol. 2019, 28, 689–702. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar] [CrossRef]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef]
- Gullian Klanian, M.; Delgadillo Díaz, M.; Sánchez Solís, M.J. Molecular characterization of histamine-producing psychrotrophic bacteria isolated from red octopus (Octopus maya) in refrigerated storage. High-Throughput 2018, 7, 25. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 873–880. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S. Liposomes loaded with betel leaf (Piper betle L.) extract: Antibacterial activity and preservative effect in combination with hurdle technologies on tilapia slices. Food Control. 2022, 138, 108999. [Google Scholar] [CrossRef]
- Feiner, G. Meat Products Handbook: Practical Science and Technology; Woodhead Publishing Limited: Boca Raton, FL, USA; Cambridge, UK, 2006; pp. 29–30, ISBN-13: 978-1-84569-172-1. [Google Scholar]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ayas, D.; Yazgan, H.; Ozogul, F.; Boga, E.K.; Ozyurt, G. The capability of rosemary extract in preventing oxidation of fish lipid. Int. J. Food Sci. Tech. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Antoniewski, M.N.; Barringer, S.; Knipe, C.; Zerby, H. Effect of a gelatin coating on the shelf life of fresh meat. J. Food Sci. 2007, 72, E382–E387. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- EC. European Commission Regulation (EC) No 1022/2008 of 17 October 2008 Amending Regulation (EC) No 2074/2005 as Regards the Total Volatile Basic Nitrogen (TVB-N) Limits. L277/18 EN Official Journal the European Union. 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1022/oj (accessed on 10 August 2022).
- Shiku, Y.; Hamaguchi, P.Y.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Tagrida, M.; Prodpran, T.; Zhang, B.; Aluko, R.E.; Benjakul, S. Liposomes loaded with betel leaf (Piper betle L.) ethanolic extract prepared by thin film hydration and ethanol injection methods: Characteristics and antioxidant activities. J. Food Biochem. 2021, 45, e14012. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S. Betel (Piper betle L.) leaf ethanolic extracts dechlorophyllized using different methods: Antioxidant and antibacterial activities, and application for shelf-life extension of Nile tilapia (Oreochromis niloticus) fillets. RSC Adv. 2021, 11, 17630–17641. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Contributions of blood and blood components to lipid oxidation in fish muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]







| Sample | Thickness (mm) | TS (MPa) | EAB (%) | L* | a* | b* | ∆E | WVP (×10−11 g m−1 s−1 Pa−1) | OP (×10−18 mol m−1 s−1 Pa−1) |
|---|---|---|---|---|---|---|---|---|---|
| PLA | 0.106 ± 0.004 a | 29.55 ± 0.54 a | 4.85 ± 1.00 a | 91.79 ± 0.01 d | −0.01 ± 0.01 d | 0.07 ± 0.01 a | - | 4.10 ± 0.18 c | 216.6 ± 11.07 e |
| GC/NF-0%BLEE | 0.115 ± 0.001 b | 32.88 ± 1.50 b | 5.00 ± 0.97 a | 89.45 ± 1.05 cd | −1.28 ± 0.06 c | 1.50 ± 0.20 a | 3.08 ± 0.84 a | 3.86 ± 0.20 bc | 25.88 ± 0.28 d |
| GC/NF-0.5%BLEE | 0.118 ± 0.001 bc | 34.03 ± 1.47 b | 5.45 ± 1.02 ab | 87.79 ± 1.19 bc | −4.63 ± 0.51 b | 5.71 ± 0.46 a | 8.37 ± 0.82 b | 3.39 ± 0.20 b | 22.59 ± 2.07 c |
| GC/NF-1%BLEE | 0.121 ± 0.001 c | 34.40 ± 1.58 b | 6.80 ± 0.18 b | 85.60 ± 1.71 b | −7.03 ± 0.51 a | 17.65 ± 1.04 b | 20.48 ± 1.95 c | 3.15 ± 0.21 ab | 10.54 ± 1.78 b |
| GC/NF-2%BLEE | 0.121 ± 0.002 c | 34.45 ± 2.65 b | 7.04 ± 2.26 b | 81.99 ± 3.02 a | −7.43 ± 0.74 a | 20.20 ± 0.89 b | 23.78 ± 0.61 c | 3.00 ± 0.01 a | 6.23 ± 0.59 a |
| Sample | Light Transmission (%) at Different Wavenumbers (nm) | Transparency Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| PLA | 0.06 | 49.75 | 69.33 | 78.79 | 83.80 | 88.21 | 88.48 | 88.60 | 0.51 ± 0.14 a |
| GC/NF-0%BLEE | 0.07 | 0.09 | 3.82 | 5.55 | 6.42 | 7.02 | 7.50 | 7.91 | 10.0 ± 0.33 b |
| GC/NF-0.5%BLEE | 0.06 | 0.06 | 2.05 | 3.03 | 3.93 | 4.87 | 5.05 | 5.28 | 11.05 ± 0.35 c |
| GC/NF-1%BLEE | 0.07 | 0.07 | 2.07 | 2.80 | 3.70 | 4.47 | 4.77 | 4.87 | 11.15 ± 0.23 c |
| GC/NF-2%BLEE | 0.07 | 0.09 | 1.91 | 2.61 | 3.67 | 4.38 | 4.63 | 4.71 | 11.21 ± 0.25 c |
| Sample | Antioxidant Activities | Antibacterial Activity (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| DPPH-RSA (µmol TE/g sample) | ABTS-RSA (µmol TE/g sample) | FRAP (µmol TE/g sample) | MCA (µmol EDTA/g sample) | S. aureus | E. coli | L. monocytogenes | P. aeruginosa | |
| PLA | ND * | ND | ND | ND | ND | ND | ND | ND |
| GC/NF-0%BLEE | 3.50 ± 1.72 a | 25.55 ± 2.22 a | ND | ND | ND | ND | ND | ND |
| GC/NF-0.5%BLEE | 23.21 ± 0.69 b | 792.5 ± 8.41b | 336.66 ± 5.50 a | 76.52 ± 3.91 a | 5.58 ± 0.50 a | 6.83 ± 0.57 a | 5.91 ± 0.68 a | 7.16 ± 0.88 a |
| GC/NF-1%BLEE | 75.89 ± 1.20 c | 1829.2 ± 7.22 c | 928.69 ± 8.72 b | 170.61 ± 1.13 b | 7.75 ± 0.63 b | 9.08 ± 0.31 b | 7.66 ± 0.72 b | 8.91 ± 0.87 b |
| GC/NF-2%BLEE | 93.49 ± 1.01d | 2817.03 ± 7.39 d | 1484.3 ± 3.13 c | 277.37 ± 0.92 c | 9.08 ± 0.68 c | 11.25 ± 0.31 c | 9.33 ± 0.89 c | 10.66 ± 0.90 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagrida, M.; Gulzar, S.; Nilsuwan, K.; Prodpran, T.; Zhang, B.; Benjakul, S. Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices. Molecules 2022, 27, 5877. https://doi.org/10.3390/molecules27185877
Tagrida M, Gulzar S, Nilsuwan K, Prodpran T, Zhang B, Benjakul S. Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices. Molecules. 2022; 27(18):5877. https://doi.org/10.3390/molecules27185877
Chicago/Turabian StyleTagrida, Mohamed, Saqib Gulzar, Krisana Nilsuwan, Thummanoon Prodpran, Bin Zhang, and Soottawat Benjakul. 2022. "Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices" Molecules 27, no. 18: 5877. https://doi.org/10.3390/molecules27185877
APA StyleTagrida, M., Gulzar, S., Nilsuwan, K., Prodpran, T., Zhang, B., & Benjakul, S. (2022). Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices. Molecules, 27(18), 5877. https://doi.org/10.3390/molecules27185877